Androstanolone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Andractim, others |
| Synonyms | Stanolone; Dihydrotestosterone; DHT; 5α-Dihydrotestosterone; 5α-DHT |
| Pregnancy category |
|
| Routes of administration | Transdermal (gel), in the cheek, under the tongue, intramuscular injection (as esters) |
| Drug class | Androgen; Anabolic steroid |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability |
Oral: Very low[1] Transdermal: 10%[1][2] Intramuscular: 100%[2] |
| Metabolism | Liver |
| Elimination half-life | Transdermal: 2.8 hours[3] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H30O2 |
| Molar mass | 290.442 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Androstanolone, or stanolone, also known as dihydrotestosterone (DHT) and sold under the brand name Andractim among others, is an androgen and anabolic steroid (AAS) medication and hormone which is used mainly in the treatment of low testosterone levels in men.[1] It is also used to treat breast development and small penis in males.[1] It is typically given as a gel for application to the skin, but can also be used as an ester by injection into muscle.[1][4]
Side effects of androstanolone include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[1] The drug is a naturally occurring androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and DHT.[1][5] It has strong androgenic effects and very weak anabolic effects, as well as no estrogenic effects.[1]
Androstanolone was discovered in 1935 and was introduced for medical use in 1953.[1][6][7][8] It is used mostly in France.[1][9] The drug has been used by weightlifters to increase performance due to its powerful androgenic properties.[10][11] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[1]
Medical uses
Androstanolone is available in pharmaceutical formulations for medical use as an androgen.[4] It is used mainly in the treatment of male hypogonadism and is specifically approved for this indication in certain countries.[12] Topical androstanolone is useful in the treatment of gynecomastia.[13] Similarly, androstanolone enanthate via intramuscular injection has been found to be effective in the treatment persistent pubertal gynecomastia.[14] The drug has also been used as a topical gel to treat small penis.[1]
Androstanolone was found to be effective in the treatment of advanced breast cancer in women in the 1950s, although it was used in very high doses and caused severe virilization.[15][16][17] Shortly thereafter, drostanolone propionate (2α-methylandrostanolone propionate) was developed for this use instead of androstanolone due to its superior pharmacokinetics and was introduced for this indication in the United States and Europe in the early 1960s.[18][19][20][21]
Side effects
Adverse effects of androstanolone are similar to those of other AAS and include androgenic side effects like oily skin, acne, seborrhea, increased facial/body hair growth, scalp hair loss, and increased aggressiveness and sex drive.[22][23] In women, androstanolone can cause partially irreversible virilization, for instance voice deepening, hirsutism, clitoromegaly, breast atrophy, and muscle hypertrophy, as well as menstrual disturbances and reversible infertility.[22][23] In men, the drug may also cause hypogonadism, testicular atrophy, and reversible infertility at sufficiently high dosages.[22][23]
Androstanolone can have adverse effects on the cardiovascular system, especially with long-term administration of high dosages.[22] AAS like androstanolone stimulate erythropoiesis (red blood cell production) and increase hematocrit levels and at high dosages can cause polycythemia (overproduction of red blood cells), which can greatly increase the risk of thrombic events such as embolism and stroke.[22] Unlike many other AAS, androstanolone is not aromatized and has no risk of estrogenic side effects like gynecomastia, fluid retention, or edema.[22][23] In addition, as it is not a 17α-alkylated AAS and is administered parenterally, androstanolone has no risk of hepatotoxicity.[22][23]
Pharmacology
Pharmacodynamics
Androstanolone is a potent agonist of the AR. It has an affinity (Kd) of 0.25 to 0.5 nM for the human AR, which is about 2- to 3-fold higher than that of testosterone (Kd = 0.4 to 1.0 nM)[24] and the dissociation rate of androstanolone from the AR is also about 5-fold slower than that of testosterone.[25] The EC50 of androstanolone for activation of the AR is 0.13 nM, which is about 5-fold stronger than that of testosterone (EC50 = 0.66 nM).[26] In bioassays, androstanolone has been found to be 2.5- to 10-fold more potent than testosterone.[24]
Unlike testosterone and various other AAS, androstanolone cannot be aromatized, and for this reason, poses no risk of estrogenic side effects like gynecomastia at any dosage.[27] In addition, androstanolone cannot be metabolized by 5α-reductase (as it is already 5α-reduced), and for this reason, is not potentiated in so-called "androgenic" tissues like the skin, hair follicles, and prostate gland, thereby improving its ratio of anabolic to androgenic effects. However, androstanolone is nonetheless described as a very poor anabolic agent.[22] This is attributed to its high affinity as a substrate for 3α-hydroxysteroid dehydrogenase (3α-HSD), which is highly expressed in skeletal muscle and inactivates androstanolone into 3α-androstanediol, a metabolite with very weak AR activity.[22] Unlike androstanolone, testosterone is very resistant to metabolism by 3α-HSD, and so is not similarly inactivated in skeletal muscle.[22]
Pharmacokinetics
Absorption
The bioavailability of androstanolone differs considerably depending on its route of administration.[1][2] Its oral bioavailability is very low, and androstanolone is considered to be ineffective by the oral route.[1] The transdermal bioavailability of androstanolone is approximately 10%.[1][2] Its bioavailability with intramuscular injection, on the other hand, is complete (100%).[2]
Distribution
The plasma protein binding of androstanolone is about 98.5 to 99.0%.[28] It is bound 50 to 80% to sex hormone-binding globulin, 20 to 40% to albumin, and less than 0.5% to corticosteroid-binding globulin, with about 1.0 to 1.5% circulating freely or unbound.[28]
Metabolism
The terminal half-life of androstanolone in the circulation (53 minutes) is longer than that of testosterone (34 minutes), and this may account for some of the difference in their potency.[29] A study of transdermal androstanolone and testosterone treatment reported terminal half-lives of 2.83 hours and 1.29 hours, respectively.[3]
Chemistry
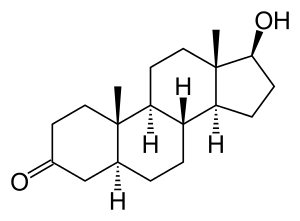
Androstanolone, also known as 5α-androstan-17β-ol-3-one or as 5α-dihydrotestosterone (5α-DHT), is a naturally occurring androstane steroid with a ketone group at the C3 position and a hydroxyl group at the C17β position.[30][31] It is the derivative of testosterone in which the double bond between the C4 and C5 positions has been reduced or hydrogenated.[30][31]
Esters
Several C17β ester prodrugs of androstanolone, including androstanolone benzoate, androstanolone enanthate, androstanolone propionate, and androstanolone valerate, have been developed and introduced for medical use as AAS. Conversely, dihydrotestosterone acetate, dihydrotestosterone butyrate, and dihydrotestosterone formate have been developed but have not been marketed.[30][32]
Derivatives
Synthetic derivatives of androstanolone (DHT) that have been developed as AAS include:[1]
|
|
History
Androstanolone was first discovered and synthesized in 1935 by Adolf Butenandt and his colleagues.[6][7] It was first introduced for medical use in 1953, under the brand name Neodrol in the United States,[8][33][34] and was subsequently marketed in the United Kingdom and other European countries.[8]
Society and culture
Generic names
When used as a drug, androstanolone is referred to as androstanolone (INN) or as stanolone (BAN) rather than as DHT.[4][30][31][9]
Brand names
Brand names of androstanolone include Anaboleen, Anabolex, Anaprotin (UK), Andractim (formerly AndroGel-DHT) (FR, BE, LU), Androlone, Apeton, Gelovit (ES), Neodrol, Ophtovital (DE), Pesomax (IT), Stanaprol, and Stanolone, among others.[4][30][31][12][35][9]
Availability
The availability of pharmaceutical androstanolone is limited; it is not available in the United States or Canada,[36][37] but it is or has been available in certain European countries, including the United Kingdom, Germany, France, Spain, Italy, Belgium, and Luxembourg.[31][12][9]
The available formulations of androstanolone include buccal or sublingual tablets (Anabolex, Stanolone), topical gels (Andractim, Gelovit, Ophtovital), and, as esters in oil, injectables like androstanolone propionate (Pesomax) and androstanolone valerate (Apeton).[4][12][35] Androstanolone benzoate (Ermalone-Amp, Hermalone, Sarcosan) and androstanolone enanthate (Anaboleen Depot) are additional androstanolone esters that are available for medical use in some countries.[30] Androstanolone esters act as prodrugs of androstanolone in the body and have a long-lasting depot effect when given via intramuscular injection.[4]
Legal status
Androstanolone, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.[38]
Research
In the early- to mid-2000s, transdermal or topical androstanolone was under development in the United States for the treatment of hypogonadism (as a form of androgen replacement therapy), male osteoporosis, and cachexia (in cancer patients) and in Australia for the treatment of benign prostatic hyperplasia (BPH).[39][40][12] It reached phase II clinical trials for hypogonadism and BPH and phase III clinical studies for cachexia but development was ultimately never completed for these indications in these specific countries.[39][40][12] Although androstanolone itself has not been approved for cachexia in any country, an orally active synthetic derivative of androstanolone, oxandrolone (2-oxa-17α-methylandrostanolone), is approved and used for this indication in the United States.[41][42]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 8, 23–25, 353–359. ISBN 978-0-9828280-1-4.
- 1 2 3 4 5 Coutts SB, Kicman AT, Hurst DT, Cowan DA (November 1997). "Intramuscular administration of 5α-dihydrotestosterone heptanoate: changes in urinary hormone profile". Clin. Chem. 43 (11): 2091–8. PMID 9365393.
- 1 2 von Deutsch DA, Abukhalaf IK, Lapu-Bula R (15 October 2003). "Anabolic Doping Agents". In Mozayani A, Raymon L. Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 510–. doi:10.1007/978-1-61779-222-9_15. ISBN 978-1-59259-654-6.
- 1 2 3 4 5 6 Hyde TE, Gengenbach MS (2007). Conservative Management of Sports Injuries. Jones & Bartlett Learning. pp. 1100–. ISBN 978-0-7637-3252-3.
- ↑ Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- 1 2 R Schnitzer (1 January 1967). Experimental Chemotherapy. Elsevier Science. pp. 156–. ISBN 978-0-323-14611-1.
- 1 2 H.-L. Krüskemper (22 October 2013). Anabolic Steroids. Elsevier. pp. 12–. ISBN 978-1-4832-6504-9.
- 1 2 3 William Andrew Publishing (2007). Pharmaceutical Manufacturing Encyclopedia. William Andrew Pub. ISBN 978-0-8155-1526-5.
- 1 2 3 4 https://www.drugs.com/international/androstanolone.html
- ↑ https://www.iwf.net/2018/05/30/public-disclosure-104/
- ↑ http://www.chicagotribune.com/news/ct-xpm-1994-12-10-9412100116-story.html
- 1 2 3 4 5 6 http://adisinsight.springer.com/drugs/800011409
- ↑ Agrawal, Sweety; Ganie, Mohd Ashraf; Nisar, Sobia (2017). "Gynaecomastia": 451–458. doi:10.1007/978-981-10-3695-8_26.
- ↑ Eberle AJ, Sparrow JT, Keenan BS (1986). "Treatment of persistent pubertal gynecomastia with dihydrotestosterone heptanoate". J. Pediatr. 109 (1): 144–9. PMID 3088241.
- ↑ Gelhorn A, Holland J, Herrmann JB, Moss J, Smelin A (1954). "An evaluation of stanolone in treatment of advanced mammary cancer". J Am Med Assoc. 154 (15): 1274–7. PMID 13151839.
- ↑ Kennedy BJ (1955). "The effect of stanolone in the treatment of advanced breast cancer". Cancer. 8 (3): 488–97. PMID 14379136.
- ↑ Segaloff A, Horwitt BN, Carabasi RA, Murison PJ, Schlosser JV (1955). "Hormonal therapy in cancer of the breast. VIII. The effect of dihydrotestosterone (androstanolone) on clinical course and hormonal excretion". Cancer. 8 (1): 82–6. PMID 13231036.
- ↑ Blackburn CM, Childs DS (1959). "Use of 2 alpha-methyl androstan-17 beta-ol, 3-one (2-methyl dihydrotestosterone) in the treatment of advanced cancer of the breast". Proc Staff Meet Mayo Clin. 34 (5): 113–26. PMID 13658242.
- ↑ Goldenberg IS, Hayes MA (1961). "Hormonal therapy of metastatic female breast carcinoma. II. 2alpha-Methyl dihydrotestosterone propionate". Cancer. 14: 705–6. PMID 13706491.
- ↑ Thomas AN, Gordan GS, Godlmanl, Lowe R (1962). "Antitumor efficacy of 2alpha-methyl dihydrotestosterone propionate in advanced breast cancer". Cancer. 15: 176–8. PMID 13920749.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1402–. ISBN 978-0-8155-1856-3.
- 1 2 3 4 5 6 7 8 9 10 William Llewellyn (2009). Anabolics. Molecular Nutrition Llc. pp. 19, 163. ISBN 978-0967930473.
- 1 2 3 4 5 Kicman, A T (2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- 1 2 Mozayani A, Raymon L (18 September 2011). Handbook of Drug Interactions: A Clinical and Forensic Guide. Springer Science & Business Media. pp. 656–. ISBN 978-1-61779-222-9.
- ↑ Grino PB, Griffin JE, Wilson JD (February 1990). "Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone". Endocrinology. 126 (2): 1165–72. doi:10.1210/endo-126-2-1165. PMID 2298157.
- ↑ Wilderer PA (1 September 2010). "Bioassays for Estrogenic and Androgenic Effects of Water Constituents". Treatise on Water Science, Four-Volume Set. Newnes. pp. 1805–. ISBN 978-0-444-53199-5.
- ↑ Malven PV (12 January 1993). Mammalian Neuroendocrinology. CRC Press. pp. 228–. ISBN 978-0-8493-8757-9.
- 1 2 Nieschlag E, Behre HM, Nieschlag S (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 61–. ISBN 978-1-107-01290-5.
- ↑ Diamanti-Kandarakis E (1999). "Current aspects of antiandrogen therapy in women". Current Pharmaceutical Design. 5 (9): 707–23. PMID 10495361.
- 1 2 3 4 5 6 Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 640–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 63–. ISBN 978-3-88763-075-1.
- ↑ Morton I, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 261–. ISBN 978-94-011-4439-1.
- ↑ Newsweek. Newsweek. 1953.
- ↑ New and Nonofficial Drugs. Lippincott. 1958.
- 1 2 List PH, Hörhammer L (12 March 2013). Chemikalien und Drogen: Teil B: R, S. Springer-Verlag. pp. 523–. ISBN 978-3-642-66377-2.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
- ↑ "Drug Product Database - Health Canada". Health Canada. Retrieved 13 November 2016.
- ↑ Steven B. Karch, MD, FFFLM (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- 1 2 http://adisinsight.springer.com/drugs/800019178
- 1 2 https://adisinsight.springer.com/drugs/800016161
- ↑ Nelms M, Sucher KP, Lacey K, Roth SL (16 June 2010). Nutrition Therapy and Pathophysiology. Cengage Learning. pp. 766–. ISBN 1-133-00809-7.
- ↑ Mantovani G (6 October 2007). Cachexia and Wasting: A Modern Approach. Springer Science & Business Media. pp. 673–. ISBN 978-88-470-0552-5.
External links
- Androstanolone (for hypogonadism and cachexia) - AdisInsight
- Androstanolone (for hypogonadism and BPH) - AdisInsight
- Androstanolone (for male osteoporosis) - AdisInsight