Cyproterone acetate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Androcur, Androcur Depot, Cyprostat, Siterone, others |
| Synonyms | SH-80714; SH-714; NSC-81430; 1α,2α-Methylene-6-chloro-17α-hydroxy-δ6-progesterone acetate; 1α,2α-Methylene-6-chloro-17α-hydroxypregna-4,6-diene-3,20-dione acetate |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular injection |
| Drug class | Steroidal antiandrogen; Progestin; Progestogen; Progestogen ester; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 88–100%[1][2] |
| Protein binding |
Albumin: 93% Free: 7%[3][4][5][6] |
| Metabolism | Hepatic (CYP3A4)[7][8] |
| Metabolites |
• 15β-OH-CPA (major)[1][9] • Cyproterone (minor)[10] • Acetic acid (minor)[10] |
| Elimination half-life |
Oral: 1.6–4.3 days[10][11][12] IM: 3–4.3 days[2][10][12] |
| Excretion |
Feces: 70%[10] Urine: 30%[10] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.006.409 |
| Chemical and physical data | |
| Formula | C24H29ClO4 |
| Molar mass | 416.942 g/mol |
| 3D model (JSmol) | |
| Melting point | 200 to 201 °C (392 to 394 °F) |
| |
| |
| (verify) | |
Cyproterone acetate (CPA), sold alone under the brand name Androcur or with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication which is used in the treatment of androgen-dependent conditions like acne, excessive hair growth, early puberty, and prostate cancer, as a component of feminizing hormone therapy for transgender women, and in birth control pills.[1][11][13][14][15] It is formulated and used both alone and in combination with an estrogen and is available for use both by mouth and by injection into muscle. CPA is taken by mouth one to three times per day and is given by injection once or twice per week.
Common side effects of high-dose CPA in men include gynecomastia (breast development) and feminization. In both men and women, side effects of CPA include low sex hormone levels, reversible infertility, sexual dysfunction, mental symptoms like depression, fatigue, and irritability, vitamin B12 deficiency, and elevated liver enzymes. At very high doses, cardiovascular complications can occur. Rare but serious adverse reactions of CPA include blood clots, liver damage, and certain types of benign brain tumors. CPA can also cause adrenal insufficiency as a withdrawal effect if it is discontinued abruptly from a high dosage. CPA blocks the effects of androgens like testosterone in the body, which it does by preventing them from interacting with their biological target, the androgen receptor (AR), and by reducing their production by the gonads and hence their concentrations in the body.[1][13][16] In addition, it has progesterone-like effects by activating the progesterone receptor (PR).[1][13] It also has weak cortisol-like effects at high doses.[1]
CPA was discovered in 1961.[17] It was originally developed as a progestin.[17] In 1965, the antiandrogenic effects of CPA were discovered.[18] CPA was first marketed, as an antiandrogen, in 1973, and was the first antiandrogen to be introduced for medical use.[19] A few years later, in 1978, CPA was introduced as a progestin in a birth control pill.[20] It has been described as a "first-generation" progestin.[21] CPA is available widely throughout the world.[22][23] An exception is the United States, where it is not approved for use.[24] CPA has been described as the prototypical antiandrogen.[25]
Medical uses
CPA is used as a progestin and antiandrogen in hormonal birth control and in the treatment of androgen-dependent conditions.[13] Specifically, CPA is used in combined birth control pills, in the treatment of androgen-dependent skin and hair conditions such as acne, seborrhea, excessive hair growth, and scalp hair loss, high androgen levels, in transgender hormone therapy, to treat prostate cancer, to reduce sex drive in sex offenders or men with paraphilias or hypersexuality, to treat early puberty, and for other uses.[26] It is used both at low doses and at higher doses.
In the United States, where CPA is not available, other medications with antiandrogenic effects are used to treat androgen-dependent conditions instead.[27] Examples of such medications include gonadotropin-releasing hormone modulators (GnRH modulators) like leuprorelin and degarelix, nonsteroidal antiandrogens like flutamide and bicalutamide, the diuretic and steroidal antiandrogen spironolactone, the progestin medroxyprogesterone acetate, and the 5α-reductase inhibitors finasteride and dutasteride.[27] The steroidal antiandrogen and progestin chlormadinone acetate is used as an alternative to CPA in Japan, South Korea, and a few other countries.
Birth control
CPA is used together with ethinylestradiol as a combined birth control pill to prevent pregnancy in women. This birth control combination has been available since 1978.[20] The formulation is taken once daily for 21 days, followed by a 7-day free interval.[28] CPA has also been available in combination with estradiol valerate (brand name Femilar) as a combined birth control pill in Finland since 1993.[29][30]
Skin and hair conditions
CPA is used as an antiandrogen to treat androgen-dependent skin and hair conditions such as acne, seborrhea, hirsutism (excessive hair growth), scalp hair loss, and hidradenitis suppurativa in women.[31][32][33][34][35][36][37] These conditions are worsened by the presence of androgens, and by suppressing androgen levels and blocking their actions, CPA improves the symptoms of these conditions. CPA is used to treat such conditions both at low doses as a birth control pill and on its own at higher doses.[31][32][33][34][38] A birth control pill containing low-dose CPA in combination with ethinylestradiol to treat acne has been found to result in overall improvement in 75 to 90% of women, with responses approaching 100% improvement.[39]
High androgen levels
CPA is used as an antiandrogen to treat high androgen levels and associated symptoms such as masculinization due to conditions like polycystic ovary syndrome (PCOS) and congenital adrenal hyperplasia (CAH) in women.[32][37][40][41][42][43]
Transgender hormone therapy
CPA is widely used as an antiandrogen and progestogen in feminizing hormone therapy for transgender women.[44][45][46][47][48][49][50] It has also been used as a puberty blocker and hence as an antiandrogen and antiestrogen to suppress puberty in transgender youth, although GnRH modulators are primarily used for this purpose.[51][52][46][53][54]
Prostate cancer
CPA is used as an antiandrogen monotherapy and means of androgen deprivation therapy in the palliative treatment of prostate cancer in men.[11][55][56][57][58][59] It is used at very high doses by mouth or by intramuscular injection to treat this disease. Antiandrogens do not cure prostate cancer, but can significantly extend life in men with the disease.[60][61][55] CPA has similar effectiveness to GnRH modulators and surgical castration, high-dose estrogen therapy (e.g., with diethylstilbestrol), and high-dose nonsteroidal antiandrogen monotherapy (e.g., with bicalutamide), but has significantly inferior effectiveness to combined androgen blockade with a GnRH modulator and a nonsteroidal antiandrogen (e.g., with bicalutamide or enzalutamide).[55][61][62][63][64] In addition, the combination of CPA with a GnRH modulator or surgical castration has not been found to improve outcomes relative to a GnRH modulator or surgical castration alone, in contrast to nonsteroidal antiandrogens.[65] Due to its inferior effectiveness, tolerability, and safety, CPA is rarely used in the treatment of prostate cancer today, having largely been superseded by GnRH modulators and nonsteroidal antiandrogens.[66][67] CPA is the only steroidal antiandrogen that continues to be used in the treatment of prostate cancer.[61]
Dose-ranging studies of CPA for prostate cancer were not performed, and the optimal dosage of CPA for the treatment of the condition has not been established.[68][69] A dosage range of oral CPA of 100 to 300 mg/day is used in the treatment of prostate cancer, but generally 150 to 200 mg/day oral CPA is used.[70][71] The combination of CPA with surgical or medical castration for prostate cancer has been found to significantly decrease overall survival compared to castration alone.[72] Hence, the use CPA as the antiandrogen component in combined androgen blockade would appear not to be advisable.[72]
Sexual deviance
CPA is used as an antiandrogen and form of chemical castration in the treatment of forms of sexual deviance such as paraphilias and hypersexuality in men.[73][74][75][76][77][78] It is used to treat sex offenders. The medication is approved in more than 20 countries for this indication and is predominantly employed in Canada, Europe, and the Middle East.[79] CPA works by decreasing sex drive and sexual arousal and producing sexual dysfunction. CPA can also be used to reduce sex drive in individuals with inappropriate sexual behaviors, such as people with intellectual disability and dementia.[80][81] The medication is also useful for treating self-harmful sexual behavior, such as masochism.[82] CPA has comparable effectiveness to medroxyprogesterone acetate in suppressing sexual urges and function but appears to be less effective than GnRH modulators like leuprorelin and has more side effects.[74]
High-dose CPA significantly decreases sexual fantasies and sexual activity in 80 to 90% of men with sexual deviance.[79] In addition, it has been found to decrease the rate of reoffending in sex offenders from 85% to 6%, with most of the reoffenses being committed by individuals who did not follow their CPA treatment prescription.[79] It has been reported that in 80% of cases, 100 mg/day CPA is adequate to achieve the desired reduction of sexuality, whereas in the remaining 20% of cases, 200 mg/day is sufficient.[83] When only a partial reduction in sexuality is desired, 50 mg/day CPA can be useful.[83] Reduced sexual desire and erectile function occurs with CPA by the end of the first week of treatment, and becomes maximal within three to four weeks.[83][84]
Early puberty
CPA is used as an antiandrogen and antiestrogen to treat precocious puberty in boys and girls.[13][41][85] However, it is not fully satisfactory for this indication because it is not able to completely suppress puberty.[86] For this reason, CPA has mostly been superseded by GnRH agonists in the treatment of precocious puberty.[41] CPA is not satisfactory for gonadotropin-independent precocious puberty.[87]
Other uses
CPA can be used at low doses in menopausal hormone therapy in combination with an estrogen to provide endometrial protection and treat menopausal symptoms.[88]
CPA is useful in the treatment of hot flashes, for instance due to androgen deprivation therapy.[62][89][90][91]
CPA is useful for suppressing the testosterone flare at the start of GnRH agonist therapy.[62][92][11][41][93] It has been used both alone and in combination with estrogens such as diethylstilbestrol for this purpose.[94]
Available forms
CPA is available in the form of oral tablets alone (higher-dose; 10 mg, 50 mg, 100 mg) or in combination with ethinylestradiol or estradiol valerate (low-dose; 2 mg CPA) and in the form of ampoules for intramuscular injection (higher-dose; 100 mg/mL, 300 mg/3 mL; brand name Androcur Depot).[95][96][97][98] The higher-dose formulations are used to treat prostate cancer and certain other androgen-related indications while the low-dose formulations which also have an estrogen are used as combined birth control pills and are used in menopausal hormone therapy for the treatment of menopausal symptoms.[97][99]
Contraindications
Side effects
Common side effects of CPA include hypogonadism and associated symptoms like demasculinization, sexual dysfunction, infertility, and osteoporosis, breast changes like gynecomastia, mental changes like depression, anxiety, fatigue, and suicidal ideation, vitamin B12 deficiency, glucocorticoid side effects like stretch marks,[101] and elevated liver enzymes. At very high doses, CPA can cause cardiovascular side effects. Rarely, CPA can cause blood clots, liver damage, excessively high prolactin levels, prolactinomas, and meningiomas. Upon discontinuation at high dosages, CPA can have withdrawal effects, namely adrenal insufficiency. Some of the side effects of CPA can be improved if it is combined with an estrogen, which prevents estrogen deficiency.[39] CPA is generally well-tolerated when used as an antiandrogen in women.[102] Little quantitative data are available on many of the potential side effects of CPA.[103]
| Side effect | Males (136 mg/day)a (n = 1248) (%) | Females (18 mg/day)a (n = 1258) (%) | Total (77 mg/day)a (n = 2506) (%) |
|---|---|---|---|
| Elevated liver enzymesb | 12.5 | 3.6 | 9.6 |
| Gynecomastia, breast pain | 4.9 | 1.0 | 2.9 |
| Overweightness, weight gain | 0.9 | 4.1 | 2.5 |
| Headache, migraine | – | 3.4 | 1.7 |
| Depression, anxiety, nightmares, mood swings | 1.0 | 1.8 | 1.4 |
| Gastrointestinal dysfunction | 0.2 | 2.3 | 1.2 |
| Thyroid dysfunction | 0.2 | 1.8 | 1.0 |
| Skin changes (pigmentation, chloasma, others) | 0.3 | 1.4 | 0.8 |
| Edema | – | 1.1 | 0.6 |
| Adrenal insufficiency or hyperplasia | 0.2 | 0.8 | 0.5 |
| Tiredness, lethargy, apathy | 0.6 | 0.6 | 0.6 |
| Alopecia, hair growth | 0.2 | 0.2 | 0.2 |
| Increased asthma attack rates | – | 0.3 | 0.2 |
| Osteoporosis | 0.2 | 0.08 | 0.2 |
| More frequently prone to attacks | 0.2 | 0.2 | 0.2 |
| Diabetes mellitus | 0.08 | 0.08 | 0.08 |
| Total (excluding elevated liver enzymes) | 9.0 | 19.1 | 14.0 |
| Notes: Data from the SExual-HOrmonal Surveillance STudy (SEHOST), a pharmacoepidemiologic active surveillance open-label uncontrolled follow-up study with historic (retrospective) accrual of patients. The sample included males and females aged 3 to 75 years with precocious puberty, hyperandrogenism, sexual deviance, and transgenderism; prostate cancer was not included. Footnotes: a = Average dosage; range less than 10 mg/day to >200 mg/day. b = Liver enzymes monitored in 1,685 individuals, including 1,131 males and 554 females. Miscellaneous: Direct link to table. Source: [106] | |||
Low hormone levels
Side effects in men resulting from the antiandrogenic and antigonadotropic properties of CPA include physical demasculinization, sexual dysfunction (including loss of libido and erectile dysfunction), impaired spermatogenesis, absence of ejaculate, and reversible infertility.[19][107] In the treatment of men with prostate cancer, CPA has been described as causing "severe" suppression of libido and erectile potency, comparable to that seen with surgical castration.[108] Due to suppression of the production of estrogens, long-term use of high-dose CPA without concomitant estrogen therapy can result in the development of osteoporosis in both sexes.[109] CPA can also sometimes cause breast changes in men including gynecomastia, breast tenderness, and galactorrhea.[19] Rates of gynecomastia of 7 to 13% have been reported.[110][111]
Depression
CPA has been associated with a potential side effect of depression in both men and women.[112] It has been reported that as many as 20 to 30% of women treated with the drug for hirsutism (dosage range 25–100 mg) may show depressive symptoms.[113][114] Also, a study found that around 20% of women treated with Dianette (which contains only 2 mg CPA) for contraceptive purposes developed depression.[115] As the antiandrogen component of transgender HRT, treatment with CPA (as well as with spironolactone to a lesser extent) has also been associated with a significantly higher rate of depressive symptomatology in transgender women relative to treatment with GnRH analogues (which are more selective in their action and are considered not to have a significant risk of depression in this patient population (with concomitant supplementation of estrogen)).[116] The depressive effects of CPA may be related to its glucocorticoid, antiandrogen, or antigonadotropic effects, as glucocorticoids, antiandrogens (in men), and GnRH analogues have all been associated with depression.[117][118][119][120] Vitamin B12 deficiency induced by CPA might also or alternatively be a critical factor.[115] Because of the side effect of depression, CPA should be used with caution in individuals with a history of the condition, especially if severe.[121]
Vitamin B12 deficiency
High-dose CPA treatment has been found to produce vitamin B12 deficiency.[122][123] Low-dose (2 mg/day) CPA in combination with ethinylestradiol has also been associated with vitamin B12 deficiency.[39] It is notable that vitamin B12 deficiency is associated with depression, anxiety, irritability, and fatigue via depletion of central monoamine neurotransmitters,[124][125] and it has been suggested that this may be involved in the adverse neuropsychiatric consequences commonly observed with CPA therapy.[115] Serum vitamin B12 monitoring and supplementation as necessary has been recommended during CPA treatment.[39][122][123]
Cardiovascular effects
At the very high doses (e.g., 300 mg/day) used to treat men with prostate cancer, CPA is associated with relatively mild cardiovascular side effects including coagulation changes[126] and blood clots (5%),[65][127] fluid retention (4%),[127] ischemic cardiomyopathy (4–40%),[128][129] and undesirable effects on serum lipid profiles.[65][130][131][132][133] Severe cardiovascular complications occur in approximately 10% at such doses and are sometimes fatal.[133][134]
Rare reactions
Liver toxicity
The most serious potential side effect of CPA is hepatotoxicity.[135] A variety of manifestations of liver disease in association with CPA treatment have been documented, including immunoallergic cytotoxic reactions, cholestasis, autoimmune hepatitis, acute hepatitis, fulminant liver failure, and cirrhosis, as well as an increased risk of hepatocellular carcinoma.[136][137] Clinical features may include jaundice, fatigue, nausea, elevated liver enzymes, hepatic necrosis and inflammation, and features of hepatic decompensation.[137] Hepatotoxicity due to CPA therapy is most common in elderly patients who are treated with high dosages of the drug for prolonged periods of time, but has also occurred in younger patients.[136] The hepatotoxicity of CPA is related to its C1α,2α methylene group.[17]
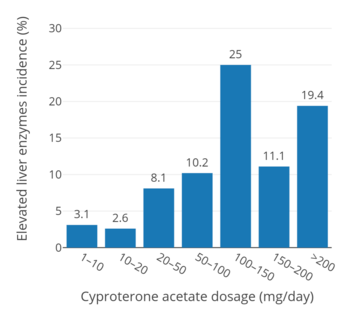
In an uncontrolled open-label active surveillance study of 1,685 healthy males and females of all ages (3 to 75 years for the full sample of 2,506 individuals) treated with CPA for an average of 6.7 years (but in 602 individuals for up to more than 10 years), elevated liver enzymes were seen in 2.6 to 3.1% of individuals at a dosage of 1 to 20 mg/day, in 8.1% of individuals at a dosage of 20 to 50 mg/day, in 10.2% of individuals at a dosage of 50 to 100 mg/day, and in 11.1 to 25.0% of individuals at a dosage of greater than 100 mg/day (up to more than 200 mg/day).[106][135][136][137] In a trial of 89 men with prostate cancer who received 50 mg/day CPA for 4 years, elevated liver enzymes occurred in 28.2%.[137][138] A study of 105 patients treated with 150 mg/day CPA reported a hepatotoxicity rate of 9.5%, with serious liver injury occurring in 3.8% (4/105).[137]
In 2002, it was reported that there were 18 case reports of CPA-associated hepatitis in the medical literature, with 6 of the cases resulting in death.[135] In addition, a review article cited a report of 96 instances of hepatotoxicity that were attributed to CPA, and 33 of these instances resulted in death.[135] A 2014 review found that 9 cases specifically of CPA-induced fulminant (sudden-onset and severe) liver failure had been reported to date, with only one of these cases not resulting in death.[137] As such, the prognosis of CPA-induced liver failure is death.[137] However, serious hepatotoxicity occurs mostly in prostate cancer patients who take very high doses of CPA, and serious liver toxicity has not been reported in transgender women.[139] All 14 reported cases of serious hepatotoxicity (acute liver failure and acute hepatitis) with CPA described in the 2014 review were in a dosage range of 100 to 300 mg/day and were in elderly men with prostate cancer (age range 65 to 92 years).[137]
The risk of hepatotoxicity and death associated with CPA treatment is reportedly the reason that CPA has not been approved by the FDA for use in the United States.[140] Patients being treated with high-dose CPA should be closely monitored with liver function tests.[141] The risk is dose-dependent, and the low doses of CPA used in birth control pills (2 mg) have been said to represent a non-significant risk.[142] However, a German woman who had been taking Diane-35 (containing 2 mg/day CPA) for contraception for 14 years died of liver cancer, and this led to a safety review by drug regulators and the eventual restriction of CPA throughout Europe for the indication of acne treatment in women.[143] In any case, liver toxicity with CPA occurs mostly in prostate cancer patients who take very high doses of the medication (200–300 mg/day), and liver toxicity has not been reported in transgender women, who usually take lower doses (25–100 mg/day).[139]
Blood clots
The combination of low-dose (2 mg) CPA in combination with ethinylestradiol (35 μg), as in combined birth control pills, presents an increased risk of venous thromboembolism (VTE).[144] Women who take contraceptive pills containing CPA have a 6- to 7-fold increased risk of developing VTE compared to women not taking a contraceptive pill, and twice the risk of women who take a contraceptive pill containing the androgenic progestin levonorgestrel.[145] At least four cases of fatal venous thromboembolism (VTE) have been attributed to low-dose CPA in combination with ethinylestradiol.[143] The progestogenic, antiandrogenic, and glucocorticoid activities of CPA are all thought to be involved in the increased risk of VTE with CPA in combination with estrogens.[1][146]
The combination of oral 100 μg/day ethinylestradiol and 100 mg/day CPA was reported to produce a 45-fold increase in the risk of VTE in 303 transgender women, with an absolute incidence of 6.3% (19 cases).[147] The risk was highly age-dependent, with a rate of VTE of 2.1% in those less than 40 years of age and of 12% in those over 40 years of age.[147] In a subsequent study of 816 transgender women in whom the same regimen was used but transdermal estradiol had become the standard therapy for those over the age of 40, the risk of VTE was still increased overall by 20-fold (45 cases, 5.5% incidence).[147] However, there was only a single case of VTE in the group of 138 transgender women treated with transdermal estradiol (0.7% incidence).[147] In accordance, the combination of transdermal estradiol and 50 mg/day cyproterone acetate appears to be relatively safe in terms of VTE risk.[147] The VTE risk was initially attributed exclusively to ethinylestradiol, and the use of ethinylestradiol has largely been abandoned in transgender women in favor of other estrogens such as estradiol because of it.[147] However, CPA is now established as increasing the risk of VTE as well, and it may have contributed also.[147] CPA should be discontinued in transgender women after sex reassignment surgery or orchiectomy to reduce the risk of VTE.[147] It should also be discontinued at least 2 weeks before undergoing surgery to reduce the risk of VTE.[147]
A large pharmacoepidemiological study in the United Kingdom using the General Practice Research Database assessed the risk of VTE with various forms of androgen deprivation therapy for prostate cancer.[148][80][149] The study had a sample of 11,199 men, of whom 229 (2.0%) experienced VTE and in whom 14% this was fatal.[148][149] The incidence rates for VTE were 3.46 for CPA monotherapy relative to nonsteroidal antiandrogen monotherapy with flutamide or bicalutamide; 3.35 for CPA monotherapy relative to GnRH agonist/orchiectomy monotherapy; 1.25 for CPA monotherapy relative to estrogen monotherapy with diethylstilbestrol or estramustine phosphate; and 0.60 for CPA monotherapy relative to combined androgen blockade with a GnRH agonist/orchiectomy and CPA.[80][103] The adjusted odds ratios for VTE were 1.00 for no treatment; 1.29 for nonsteroidal antiandrogen therapy; 3.35 for combined androgen blockade with CPA and a GnRH agonist/orchiectomy; 5.23 for CPA monotherapy; and 5.67 for estrogen monotherapy.[148][149][150] The adjusted odds ratios for VTE of different dosages of CPA with or without a GnRH agonist relative to GnRH agonist monotherapy were 3.49 for 25 or 50 mg/day, 4.93 for 100 or 150 mg/day, and 4.54 for greater than or equal to 200 mg/day.[149] In addition to CPA and other medications used to treat prostate cancer, metastatic prostate cancer is itself a risk factor for VTE.[147]
Benign brain tumors
When used in combination with an estrogen, high-dose CPA has been associated, albeit very rarely, with two different types of benign brain tumors: prolactinomas and meningiomas.[151][152] The combination has been associated with a 400-fold increased incidence of hyperprolactinemia (high prolactin levels) in transgender women.[153][154] Hyperprolactinemia caused by the combination is related to prolactinomas, benign tumors of the pituitary gland that secrete prolactin.[154] Estrogen alone has been associated only with single case reports of prolactinoma in this population.[153] The combination has also been associated with the incidence and aggravation of meningiomas, usually-benign tumors of the meninges.[25][155] For this reason, high-dose CPA is contraindicated in people with meningioma or a history of meningoma.[7][121] Benign brain tumors caused by high-dose CPA in combination with an estrogen can cause visual disturbances or in severe cases complete blindness due to compression of the optic nerve and/or chiasm.[151]
Long-term effects
The Women's Health Initiative and other clinical studies observed a significantly increased risk of breast cancer, blood clots, and cardiovascular disease when 2.5 mg/day medroxyprogesterone acetate, a progestin closely related to CPA, was added to 0.625 mg/day conjugated estrogens in postmenopausal women.[156][157] In terms of ovulation inhibition, the effective dosage of CPA is 1.0 mg/day while that of medroxyprogesterone acetate is 10 mg/day.[1][158] Based on ovulation inhibition, a dosage of 50 mg/day cyproterone acetate has on the order of 200 times the progestogenic potency of 2.5 mg/day medroxyprogesterone acetate.[1][158] In addition to its progestogenic activity, CPA produces androgen and estrogen deficiency when used as a monotherapy,[76] and this influences health as well.[159][160][161] The health effects of CPA with long-term therapy have not been well-studied. A meta-analysis of high-dose CPA for the treatment of prostate cancer in men found that CPA was associated with a slight excess of non-prostate cancer deaths.[70] In addition, the combination of CPA with surgical or medical castration for prostate cancer has been found to significantly decrease overall survival relative to castration alone.[72]
| Event | Relative Risk CEEs/MPA vs. placebo at 5.2 years (95% CI*) | Placebo (n = 8102) | CEEs/MPA (n = 8506) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute Risk per 10,000 Women-Years | |||||||||
| Coronary heart disease events Non-fatal myocardial infarction Coronary heart disease death | 1.29 (1.02–1.63) 1.32 (1.02–1.72) 1.18 (0.70–1.97) | 30 23 6 | 37 30 7 | ||||||
| Invasive breast cancera | 1.26 (1.00–1.59) | 30 | 38 | ||||||
| Stroke | 1.41 (1.07–1.85) | 21 | 29 | ||||||
| Pulmonary embolism | 2.13 (1.39–3.25) | 8 | 16 | ||||||
| Colorectal cancer | 0.63 (0.43–0.92) | 16 | 10 | ||||||
| Endometrial cancer | 0.83 (0.47–1.47) | 6 | 5 | ||||||
| Hip fracture | 0.66 (0.45–0.98) | 15 | 10 | ||||||
| Death due to causes other than above | 0.92 (0.74–1.14) | 40 | 37 | ||||||
| Global Indexb | 1.15 (1.03–1.28) | 151 | 170 | ||||||
| Deep vein thrombosisc | 2.07 (1.49–2.87) | 13 | 26 | ||||||
| Vertebral fracturesc | 0.66 (0.44–0.98) | 15 | 9 | ||||||
| Other osteoporotic fracturesc | 0.77 (0.69–0.86) | 170 | 131 | ||||||
| WHI = Women's Health Initiative. CEEs = Conjugated estrogens. MPA = Medroxyprogesterone acetate. a = Includes metastatic and non-metastatic breast cancer with the exception of in situ breast cancer. b = A subset of the events was combined in a "global index", defined as the earliest occurrence of coronary heart disease events, invasive breast cancer, stroke, pulmonary embolism, endometrial cancer, colorectal cancer, hip fracture, or death due to other causes. c = Not included in Global Index. * = Nominal confidence intervals unadjusted for multiple looks and multiple comparisons. Sources:[162][163] | |||||||||
Withdrawal
Adrenal insufficiency
Abrupt withdrawal of CPA can be harmful, and the package insert from Schering AG recommends the daily dose be reduced by no more than 50 mg at intervals of several weeks. The concern is the manner in which CPA affects the adrenal glands. Due to its glucocorticoid activity, high levels of CPA may reduce ACTH, resulting in adrenal insufficiency if discontinued abruptly. In addition, although CPA reduces androgen production in the gonads, it can increase the production of adrenal androgens, in some cases resulting in an overall rise in testosterone levels.[164] Thus, the sudden withdrawal of CPA could result in undesirable androgenic effects. This is a particular concern because androgens, especially DHT, suppress adrenal function, further reducing corticosteroid production.[165]
Suppression of adrenal function and reduced response to adrenocorticotropic hormone (ACTH) have been reported with CPA treatment. As a result, adrenal insufficiency and hence low cortisol and aldosterone levels and ACTH responsiveness can occur upon discontinuation of CPA. Low aldosterone levels may lead to hyponatremia (sodium loss) and hyperkalemia (excess potassium). Patients taking CPA should have their cortisol levels and electrolytes monitored, and if hyperkalemia develops, should reduce the consumption of foods with high potassium content or discontinue the medication.
Overdose
CPA is relatively safe in acute overdose.[166] It is used at very high doses of up to 300 mg/day by mouth and 700 mg per week by intramuscular injection.[166][167] For comparison, the dose of CPA used in birth control pills is 2 mg/day.[168] There have been no deaths associated with CPA overdose.[166] There are no specific antidotes for CPA overdose, and treatment should be symptom-based.[166] Gastric lavage can be used in the event of oral overdose within the last 2 to 3 hours.[166]
Interactions
Inhibitors and inducers of the cytochrome P450 enzyme CYP3A4 may interact with CPA.[166] Examples of strong CYP3A4 inhibitors include ketoconazole, itraconazole, clotrimazole, and ritonavir, while examples of strong CYP3A4 inducers include rifampicin, rifampin, phenytoin, carbamazepine, phenobarbital, and St. John's wort.[166] Certain anticonvulsant medications can substantially reduce levels of CPA, by as much as 8-fold.[34]
Pharmacology
Pharmacodynamics
CPA has antiandrogenic activity,[1][169] progestogenic activity,[1][169] weak partial glucocorticoid activity,[170] weak steroidogenesis inhibitor activity,[171] and agonist activity at the pregnane X receptor.[172][173][174] It has no estrogenic or antimineralocorticoid activity.[1]
| Progestogen | PR | AR | ER | GR | MR |
|---|---|---|---|---|---|
| Cyproterone acetate | 90 | 6 | 0 | 6 | 8 |
| Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, and aldosterone for the MR. Source: [1] | |||||
Antiandrogenic activity
| Compound | RBA (%) |
|---|---|
| Metribolone | 100 |
| Dihydrotestosterone | 85 |
| Cyproterone acetate | 7.8 |
| Bicalutamide | 1.4 |
| Nilutamide | 0.9 |
| Hydroxyflutamide | 0.57 |
| Flutamide | <0.0057 |
CPA is a potent competitive antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone (DHT).[169] It is reportedly the most potent AR antagonist of the steroidal antiandrogens,[176] out of hundreds of other compounds.[20] In accordance, CPA has the highest antiandrogenic activity of any other clinically used progestin.[177][178] CPA directly blocks endogenous androgens like testosterone and DHT from binding to and activating the AR, and thus prevents them from exerting their androgenic effects, such as masculinization and prostate gland growth, in the body.[57][177]
The antiandrogenic activity of CPA is dose-dependent.[177][179] Although CPA is a very potent antiandrogen, high doses of CPA are nonetheless required for clinically important AR antagonism.[17][179] The clinical antiandrogenic efficacy of birth control pills containing CPA, which have only low doses of CPA in them, often can't be distinguished from that of birth control pills containing other progestins.[17] It is likely that the antiandrogenic effects of CPA-containing birth control pills are due mostly to the ethinylestradiol component, rather than the small doses of CPA present in them.[107][179] A dosage of 100 mg/day CPA can achieve a 65 to 70% reduction in sebum excretion rate in men within 4 weeks of treatment, but dosages of 10 mg/day CPA or less are said to have a negligible effect.[107] It has been stated that oral doses of CPA of at least 300 mg/day may achieve a combined androgen blockade action in the treatment of prostate cancer.[130] Although higher doses of CPA are necessary for adequate systemic AR antagonistic activity, it is notable that even low doses of oral CPA would appear to be able to significantly antagonize AR signaling in the liver in women.[180] This is probably related to the hepatic first-pass effect of oral administration, and is evidenced by the fact that whereas combined birth control pills containing CPA increase SHBG levels by 300 to 400%, combined birth control pills containing various other progestins, with either androgenic or antiandrogenic activity, increase SHBG levels by only 50 to 300%.[180] (Estrogens stimulate hepatic SHBG production while androgens inhibit hepatic SHBG production, and vice-versa for their antagonists.)[1][181] The antiandrogenic activity of CPA may also be responsible for the relatively greater risk of venous thromboembolism with CPA-containing birth control pills compared to those containing other progestins.[182]
In rats, a dosage of CPA of 25 mg/kg/day results in complete regression of prostate gland growth in gonadally intact males.[57] The equivalent dosage in humans, on the basis of body surface (conversion factor from rat to human of 6), is 4 mg/kg/day, or approximately 300 mg/day CPA for a 75 kg (165 lb) man.[57] Other techniques for determining the dosage of CPA have validated this extrapolation, for instance affinity studies and prostatic CPA levels.[57] The affinity of CPA for the AR is around 20-fold lower than that of DHT, and an excess of CPA levels of around 20 to 30 times those of DHT would hence be expected to maximally neutralize androgen signaling.[57] In accordance, it has been experimentally determined that a 3- to 10-fold excess of CPA can inhibit the effects of an "androgen" (probably testosterone or DHT) by 50%.[34] High-dose CPA has been found to achieve prostatic levels that are at least 30-fold those of DHT.[57] One study found that levels of CPA in the prostate gland in men being treated with 200 mg/day oral CPA were about 28 times those of DHT.[57]
In accordance with such findings, high-dose CPA shows equivalent effects on the prostate gland in men as high-dose diethylstilbestrol or buserelin, which both achieve castrate levels of testosterone.[57] However, a lower dosage of 50 mg/day CPA has been found to produce a reduction in prostate volume in men with benign prostatic hyperplasia that is reportedly comparable to that observed with surgical or medical castration.[57] In accordance, the dosage of CPA that achieves complete inhibition of the secretory function of the healthy prostate gland is around 50 to 100 mg/day, which is less than the dosage of 200 to 300 mg/day CPA that is used to treat prostate cancer.[84] It has been said that in combined androgen blockade regimens with castration and CPA as the AR antagonist for prostate cancer, due to the marked reduction in androgen levels, lower dosages of CPA than those used as a monotherapy would seem to be equally effective.[57] Relative to the 200 to 300 mg/day dosage of CPA used as a monotherapy in prostate cancer, the recommended dosage in combined androgen blockade is 100 to 200 mg/day, which it has been said should be more than necessary to inhibit the effects of the adrenal androgens that remain in castrated men.[84]
Significant spermatogenesis occurs with 50 mg/day CPA, but is significantly reduced compared to normal.[83] At a dosage of 200 mg/day, CPA has been found to produce azoospermia (sperm count of less than 1 million/mL) in men within 8 to 10 weeks of treatment.[83] However, fertility is generally lost even at a lower dosage of CPA of 100 mg/day because there is complete inhibition of the accessory sex glands and hence an absence of semen production and ejaculate upon orgasm.[83][84][107] Ejaculate volume decreases to almost zero after 6 weeks of high-dose CPA therapy.[107] The effects of CPA on fertility are completely reversible.[84] This has been demonstrated in clinical studies of male adolescents and adults treated with CPA continuously for 6 to 7 years.[84] The antiandrogenic effects of CPA in general appear to be greater than those of surgical or medical castration; high-dose CPA therapy greatly reduces libido and erectile potency and results in an absence of ejaculate, whereas in castrated adult men there is relatively little loss of sex drive and erectile function in most cases and it is still possible to produce ejaculate.[107] This is in spite of a much greater reduction in androgen levels with surgical or medical castration than with CPA, which demonstrates the potent AR antagonistic activity of CPA.[107]
CPA, like spironolactone and other steroidal antiandrogens such as chlormadinone acetate and megestrol acetate, is actually not a pure antagonist of the AR – that is, a silent antagonist – but rather is a very weak partial agonist.[17][169][183][184][185][186] Clinically, CPA generally functions purely as an antiandrogen, as it displaces much more efficacious endogenous androgens such as testosterone and DHT from interacting with the receptor and thus its net effect is virtually always to lower physiological androgenic activity.[57][88] But unlike silent antagonists of the AR like nonsteroidal antiandrogens such as flutamide, bicalutamide, and enzalutamide, CPA, by virtue of its slight intrinsic activity at the AR, may be unable to abolish androgenic activity in the body, which may persist to an extent in some tissues such as the prostate gland.[17] In accordance with its albeit weak capacity for activation of the AR, CPA has been found to stimulate androgen-sensitive carcinoma growth in the absence of other androgens, an effect which could be blocked by co-treatment with flutamide.[17][184][185] As a result, CPA may not be as effective in the treatment of certain androgen-sensitive conditions such as prostate cancer compared to nonsteroidal antiandrogens with a silent antagonist profile at the AR.[169][187] Indeed, CPA has never been found to extend life in prostate cancer patients when added to castration relative to castration alone, unlike nonsteroidal antiandrogens.[65] In any case, the very weak androgenic activity of CPA and its significance in humans has been contested.[57]
A paradoxical effect occurs with certain prostate cancer cells which have genetic mutations in their ARs. These altered ARs can be activated, rather than inhibited, by CPA. In such cases, withdrawal of CPA may result in a reduction in cancer growth, rather than the reverse.[188] This is known as antiandrogen withdrawal syndrome.
CPA may also have a slight direct inhibitory effect on 5α-reductase, though the evidence for this is sparse and conflicting.[189][190][191] In any case, the combination of CPA and finasteride, a well-established, selective 5α-reductase inhibitor, has been found to result in significantly improved effectiveness in the treatment of hirsutism relative to CPA alone, suggesting that if CPA does have any direct inhibitory effects on 5α-reductase, they must be far from maximal.[192][193]
In addition to its AR antagonistic activity and suppression of gonadal sex-hormone production, high-dose CPA has been found to suppress the levels of adrenal androgens such as dehydroepiandrosterone sulfate (DHEA-S), which is due to exertion of negative feedback by CPA on adrenocorticotropic hormone (ACTH) secretion via the glucocorticoid activity of CPA.[17][34][39]
Progestogenic activity
CPA is a highly potent progestogen.[168] It is described as the most potent progestin of the 17α-hydroxyprogesterone group, being about 1,200-fold more potent than hydroxyprogesterone acetate, 12-fold more potent than medroxyprogesterone acetate, and 3-fold more potent than chlormadinone acetate in animal bioassays.[107][194] Based on results in the animal bioassays, CPA has also been said to be the most potent progestin known, with 1,000 times the potency of progesterone.[107] With oral administration in humans however, CPA is distinctly less potent as a progestogen than various other progestins such as the 19-nortestosterone derivatives.[1] The effective dosage needed to inhibit ovulation in women (i.e., to act as a contraceptive) is 1 mg/day,[1] and the medication is marketed as a contraceptive (combined with low-dose ethinylestradiol) at a dosage of 2 mg/day.[168][179] For comparison, the ovulation-inhibiting dosage of levonorgestrel is 50 µg/day.[1] CPA is said to be equipotent as a progestogen and antiandrogen.[194]
Through its action as a progestogen, CPA has been found to significantly increase prolactin secretion and to induce extensive lobuloalveolar development of the mammary glands of female rhesus macaques.[195] In accordance, a study found that CPA, in all cases, induced full lobuloalveolar development of the breasts in transgender women treated with the drug in combination with estrogen for a prolonged period of time.[196][197][198] Pregnancy-like breast hyperplasia was observed in two of the subjects.[198] In contrast, the same study found that men with prostate cancer treated with a non-progestogenic antiandrogen like flutamide or bicalutamide and no estrogen produced moderate but incomplete lobuloalveolar development of the breasts.[196] Based on the above research, it was concluded by the study authors that combined estrogenic and progestogenic action is required in transgender women for fully mature female-like histologic breast development (i.e., that includes complete lobuloalveolar maturation).[196][197] Also, it was observed that lobuloalveolar maturation reverses upon discontinuation of CPA after surgical castration, similarly to the case of mammary gland involution in postpartum women, indicating that continued progestogen treatment is necessary to maintain the histology.[196] It should be noted however that although these findings may have important implications in the context of lactation and breastfeeding, epithelial tissue accounts for approximately only 10% of breast volume (with the bulk of the breasts (80–90%) being represented by stromal or adipose tissue,[199][200][201][202] and it is uncertain to what extent, if any, that development of lobuloalveolar structures (a type of epithelial tissue) contributes to breast size or shape.[203]
Antigonadotropic effects
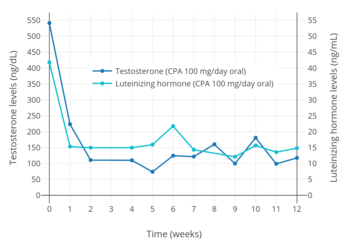
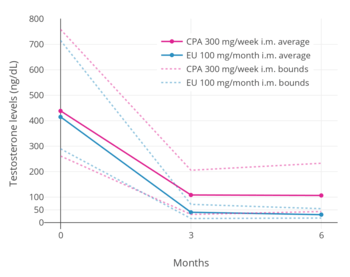
CPA has potent antigonadotropic effects via activation of the PR.[13][108][168][169] It blunts the gonadotropin releasing hormone (GnRH)-induced secretion of gonadotropins, and accordingly, markedly suppresses circulating levels of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) at sufficiently high dosages.[13][206] Consequently, levels of progesterone, androstenedione, testosterone, DHT, and estradiol are also markedly lowered at sufficiently high dosages, while an elevation in sex hormone-binding globulin (SHBG) and prolactin levels is observed.[207][208][209][210][211] CPA is able to lower circulating testosterone concentrations by 70 to 80% in men at sufficiently high dosages.[107][212] However, in spite of strong suppression of testosterone levels, CPA, at least by itself (e.g., without estrogen), is not usually able to reduce testosterone levels into the castrate/female range (<50 ng/dL) at any dosage, and testosterone levels generally remain just above it at circulating levels of roughly 50 to 100 ng/dL.[107][213] CPA has been found to maximally suppress testosterone and estradiol levels in young men within 7 days of continuous administration.[11] Following discontinuation of CPA, the recovery of testosterone levels is variable and may require 14 days to 6 months for completion.[34]
Oral CPA has been studied at low dosages of 5 to 20 mg/day as a potential male hormonal contraceptive.[214][215] A dosage of as low as 10 mg/day oral CPA was found to suppress circulating testosterone levels in men by 50 to 70%.[207][216][217][218] For comparison, the suppression of circulating testosterone levels in men with a high dosage of 100 mg/day oral CPA was 77% and with a very high dosage of 300 mg/week intramuscular CPA was 76%.[205][219] Another study found no difference in suppression of circulating testosterone levels in transgender women by the combination of estrogen and 25 mg/day oral CPA (95% suppression) and the combination of estrogen and 50 mg/day oral CPA (94% suppression).[220] The estrogen used was moderate-dose oral or transdermal estradiol (mean 3.3 mg/day oral, 3.4 g/day gel, 95.6 µg/day patches).[220]
A high dosage of CPA given for 7 days prior to initiation of GnRH agonist therapy was found to prevent the GnRH agonist-induced flare in testosterone levels.[11] CPA should be given continuously for at least a week prior to GnRH agonist initiation for an optimal preventative effect on the testosterone flare.[11]
CPA is much more potent than nonsteroidal antiandrogens like flutamide and bicalutamide in gonadally intact male animals, which is due to its antigonadotropic effects and consequent suppression of testosterone levels.[84] Conversely, nonsteroidal antiandrogens are relatively more efficacious than CPA in castrated animals, due to their superior AR antagonistic activity.[84]
Glucocorticoid activity
CPA is an agonist of the glucocorticoid receptor (GR), and has weak and partial glucocorticoid activity at high doses.[1][34][84] In animals, CPA suppresses the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland, suppresses the production of corticosteroids like cortisol and corticosterone by the adrenal cortices, and decreases the weights of the adrenal glands and thymus.[84] Conversely however, CPA shows no anti-inflammatory or eosinophilic effects in animals.[84] As such, CPA, as well as related antiandrogens, show only some of the typical effects of glucocorticoids.[84] Clinically, the glucocorticoid effects of CPA appear to be relevant only at high doses in people with small body sizes (CPA exposure of more than 80–100 mg/m2), namely in the treatment of children with precocious puberty.[84] No signs of secondary adrenal insufficiency have been observed with CPA.[84]
Due to negative feedback on the hypothalamic–pituitary-adrenal (HPA) axis, administration of exogenous glucocorticoids such as prednisone and dexamethasone suppress the secretion of adrenocorticotropic hormone (ACTH) from the pituitary gland and the production of cortisol from the adrenal glands, resulting in adrenal suppression and atrophy and, upon discontinuation of the glucocorticoid, temporary adrenal insufficiency. Similarly, albeit relatively weakly, CPA has the ability to reduce ACTH and cortisol levels and produce adrenal gland shrinkage, as well as, upon discontinuation, adrenal insufficiency, in both animals and humans, indicating that it possesses weak glucocorticoid properties.[221][222][223][224][225][226][227] Paradoxically however, in vitro, CPA is an antagonist of the glucocorticoid receptor (GR)[170][228][229] and a suppressor of adrenal cortisol and corticosterone production by inhibiting the enzymes 3β-hydroxysteroid dehydrogenase and 21-hydroxylase,[223][230][231][232] which are antiglucocorticoid actions. This paradox may be explained by the fact that certain active metabolites of CPA, such as its major metabolite 15β-hydroxycyproterone acetate (which is present at serum levels approximately twice those of CPA in humans[9]),[233] are, contrarily, agonists of the GR,[234] and it can be assumed that their glucocorticoid actions overall significantly outweigh the simultaneous antiglucocorticoid actions of CPA. Both cyproterone and CPA, via their metabolites, have been found to possess glucocorticoid effects, and based on studies in mice, it has been suggested that CPA has approximately 1/5th the potency of prednisone as a glucocorticoid.[235]
While various studies have clearly shown reduced cortisol and ACTH levels and ACTH responsiveness in humans with CPA treatment, some studies contradict these findings and report no such effects even with high dosages.[234][236][237][238][239]
Megestrol acetate, medroxyprogesterone acetate, and chlormadinone acetate, steroidal progestins and close analogues of CPA, all similarly possess glucocorticoid properties and the potential for producing adrenal insufficiency upon their discontinuation.[240][241]
Other activities
Because CPA does not bind to the ER, and because it suppresses estrogen production via its action as an antigonadotropin, the medication produces no general estrogenic effects (direct or indirect) and is potently functionally antiestrogenic at sufficient dosages.[1] However, androgens strongly antagonize the actions of estrogens in the breasts, so CPA can produce an indirect estrogenic effect of slight gynecomastia in males via its action as an antiandrogen. In any case, the incidence and severity of this side effect is less than that observed with nonsteroidal antiandrogens such as flutamide and bicalutamide, which, in contrast, do not lower estrogen levels (and actually can increase them).[76][242]
CPA has been found to bind non-selectively to the opioid receptors, including the μ-, δ-, and κ-opioid receptor subtypes, albeit very weakly relative to its other actions (IC50 for inhibition of [3H]diprenorphine binding = 1.62 ± 0.33 µM).[243][244] It has been suggested that activation of opioid receptors could have the potential to explain the side effect of sedation sometimes seen at high doses with CPA treatment or its effectiveness in the treatment of cluster headaches.[243]
Pharmacokinetics
Absorption
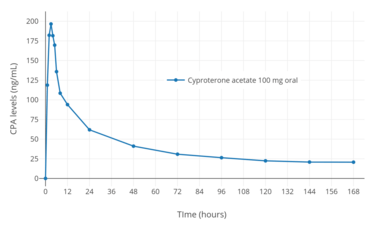
The oral bioavailability of CPA is 88 to 100%.[1][2] The absorption of oral CPA is slow but complete, and the medication is not subject to a significant first-pass effect.[34][166] The mean absorption half-life of oral CPA is about 1.5 hours.[34] Steady-state levels of CPA occur with oral CPA after about 8 days of continuous administration, with a 2- to 3-fold gradual accumulation in CPA levels.[34] Oral CPA is taken daily and intramuscular CPA is administered weekly or biweekly.[167]
Following a single low oral dose of 2 mg CPA in combination with 35 or 50 µg ethinylestradiol in premenopausal women, mean peak levels of CPA of 7.2 to 15.2 ng/mL (17–36.5 nmol/L) have been recorded after 1.6 to 3.7 hours.[177][246][247][248][249] In healthy men, a single high oral dose of 100 mg CPA produced maximal CPA levels of 254 ng/mL (609 nmol/L) after 2.6 hours.[245] Similarly, in healthy young women, a single high oral dose of 100 mg CPA resulted in peak CPA levels of 255 ng/mL (612 nmol/L) within 2 to 3 hours.[2] During continuous treatment with high oral doses of CPA in women with hirsutism, levels of CPA were 199 to 228 ng/mL (477–547 nmol/L) with 50 mg/day CPA and were 436 to 520 ng/mL (1050–1250 nmol/L) with 100 mg/day CPA.[250]
After a single intramuscular injection of 300 mg CPA in healthy young women, maximal levels of CPA of 191 ng/mL (458 nmol/L) occurred after 2 to 3 days.[2] During continuous weekly intramuscular injections of CPA in men with prostate cancer, mean levels of CPA roughly doubled from 170 ng/mL (408 nmol/L) after the first injection to 310 ng/mL (744 nmol/L) after the fifth injection, and was projected to increase to 350 to 400 ng/mL (840–960 nmol/L) after around 8 to 12 injections.[208] The area-under-the-curve (AUC; total exposure) levels of CPA with 100 mg/day oral CPA and 300 mg/week intramuscular CPA may be approximately equivalent.[208]
Distribution
With oral CPA, there is a probable distribution phase of CPA into tissues which lasts about 12 hours and has a half-life of 3 hours.[34] CPA is very lipophilic, and it is sequestered into fat, which provides a depot effect.[11][34][177] CPA crosses the blood–brain barrier, which is evidenced by the suppression of gonadotropin secretion that is observed during therapy with it (the site of action of this effect being the pituitary gland, a part of the brain).[251] In terms of plasma protein binding, CPA does not bind to SHBG or corticosteroid-binding globulin[252] and is instead bound exclusively to albumin (93%), with the remainder (7%) circulating free or unbound.[1][3][4][5][6] The affinity of CPA for SHBG is very low at about 0.006% of that of testosterone or DHT.[34]
Metabolism
CPA is metabolized primarily by hydroxylation via CYP3A4, forming the major active metabolite 15β-hydroxycyproterone acetate.[1][9] This metabolite circulates at concentrations approximately twice those of CPA, and has similar antiandrogen activity to that of CPA but only 10% of its activity as a progestogen.[1][9][253][254] As a result, the co-administration of CPA with drugs which inhibit CYP3A4 may increase its potency as a progestogen.[3]
A portion of ingested CPA is metabolized by hydrolysis into cyproterone and acetic acid.[255] However, unlike many other steroid esters, CPA is not extensively hydrolyzed, and much of the pharmacological activity of the drug is attributable to CPA itself in its unchanged form.[141] Cyproterone has approximately one-third the potency of CPA as an antiandrogen[256] and is devoid of progestogenic activity.[107]
The elimination half-life of oral CPA is relatively long at approximately 1.6 to 1.8 days (38 to 43 hours), but possibly as long as 4.3 days (100 hours).[1][11][69] The elimination half-life of CPA is prolonged in obese patients, which may be due to relatively greater storage of CPA in fat.[34] The elimination half-life of CPA is also longer in older individuals; it is approximately twice as long in elderly men than in younger men (95 hours and 45 hours, respectively).[245] When given via depot intramuscular injection, the medication has an elimination half-life of 3 to 4.3 days.[2][10][12]
Excretion
Chemistry
CPA, also known as 1α,2α-methylene-6-chloro-17α-acetoxy-δ6-progesterone or as 1α,2α-methylene-6-chloro-17α-hydroxypregna-4,6-diene-3,20-dione acetate, is a synthetic pregnane steroid and an acetylated derivative of 17α-hydroxyprogesterone.[107][257] It is structurally related to other 17α-hydroxyprogesterone derivatives such as chlormadinone acetate, hydroxyprogesterone caproate, medroxyprogesterone acetate, and megestrol acetate.[257]
Synthesis
Chemical syntheses of CPA have been published.[95][258][259] The following is one such synthesis:[260][261]
History
CPA was first synthesized in 1961 by Rudolf Wiechert, a Schering employee, and together with Friedmund Neumann in Berlin, they filed for a patent for CPA as "progestational agent" in 1962.[17][262] The antiandrogenic activity of CPA was discovered shortly thereafter.[84] CPA was initially developed as a progestogen for the prevention of threatened abortion.[84] As part of its development, CPA was assessed for androgenic activity to ensure that it would not produce teratogenic effects in female fetuses.[84] It was administered to pregnant rats and its effects on the rat fetuses were studied.[84] However, the experiment was complicated by the fact that all of the rat pups born appeared to be female.[84] After 20 female rat pups in a row had been counted however, it was clear that this was no coincidence.[84] The rat pups were further evaluated and it was found that, in terms of karyotype, about 50% were actually males.[84] CPA had feminized the male rat pups, and the antiandrogenic activity of CPA had been discovered.[84] A year after patent approval in 1965, Neumann published the first evidence of CPA's antiandrogenic effect in rats; he reported an "organizational effect of CPA on the brain".[18] During the same year, in 1966, a publication by a group in Lund, Sweden described for the first time that prenatal exposure to CPA caused urogenital malformations in male rats.[263] CPA started being used in animal experiments around the world to investigate how antiandrogens affected fetal sexual differentiation.
The first clinical use of CPA in the treatment of sexual deviance was reported in 1967.[264] In 1973, CPA was first approved in Europe, under the brand name Androcur.[258][265] Until the development of leuprorelin, CPA was one of the few drugs used to treat precocious puberty. CPA was first marketed in combination with ethinylestradiol as an oral contraceptive in 1978 under the brand name Diane.[20]
Along with the steroidal benorterone (17α-methyl-B-nortestosterone; SKF-7690), cyproterone, BOMT (Ro 7-2340), and trimethyltrienolone (R-2956) and the nonsteroidal flutamide and DIMP (Ro 7-8117), CPA was one of the first antiandrogens to be discovered and studied.[39][266][267][268]
Society and culture
Generic names
The English and generic name of CPA is cyproterone acetate and this is its USAN, BAN, and JAN.[22][23][270][271] The English and generic name of unacetylated cyproterone is cyproterone and this is its INN and BAN,[270][271][272] while cyprotérone is the DCF and French name and ciproterone is the DCIT and Italian name.[22][23] The name of unesterified cyproterone in Latin is cyproteronum, in German is cyproteron, and in Spanish is ciproterona.[22][23] These names of cyproterone correspond for CPA to acétate de cyprotérone in French, acetato de ciproterona in Spanish, ciproterone acetato in Italian, cyproteronacetat in German, cyproteronacetaat in Dutch, and ciproteron acetat in Slavic.
CPA is also known by the developmental code names SH-80714 and SH-714, while unacetylated cyproterone is known by the developmental code names SH-80881 and SH-881.[22][23][270][271]
Brand names
CPA is marketed under brand names including Androcur, Androcur Depot, Androcur-100, Androstat, Asoteron, Cyprone, Cyproplex, Cyprostat, Cysaxal, Imvel, and Siterone.[22][23] When CPA is formulated in combination with ethinylestradiol, it is also known as co-cyprindiol, and brand names for this formulation include Andro-Diane, Bella HEXAL 35, Chloe, Cypretil, Cypretyl, Cyproderm, Diane, Diane Mite, Diane-35, Dianette, Dixi 35, Drina, Elleacnelle, Estelle, Estelle-35, Ginette, Linface, Minerva, Vreya, and Zyrona.[22][23] CPA is also marketed in combination with estradiol valerate as Climen, Climene, Elamax, and Femilar.[22]
Availability
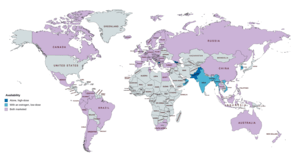
CPA is widely available throughout the world, and is marketed in almost every developed country,[273] with the notable major exceptions of the United States and Japan.[22][23][24][274][275] In almost all countries in which CPA is marketed, it is available both alone and in combination with an estrogen in birth control pills.[22][274][275] CPA is marketed widely in combination with both ethinylestradiol and estradiol valerate.[22][23][274][275] CPA-containing birth control pills are available in South Korea, but CPA as a standalone medication is not marketed in this country.[22][23][274][275] In Japan and South Korea, the closely related antiandrogen and progestin chlormadinone acetate, as well as other medications, are used instead of CPA.[276] Specific places in which CPA is marketed include the United Kingdom, elsewhere throughout Europe, Canada, Australia, New Zealand, South Africa, Latin America, and Asia.[22][23][274][275] CPA is not marketed in most of Africa and the Middle East.[22][23][274][275]
Generation
Progestins in birth control pills are sometimes grouped by generation.[277][278] While the 19-nortestosterone progestins are consistently grouped into generations, the pregnane progestins that are or have been used in birth control pills are typically omitted from such classifications or are grouped simply as "miscellaneous" or "pregnanes".[277][278] In any case, CPA has been described as a "first-generation" progestin similarly to closely related progestins like chlormadinone acetate, medroxyprogesterone acetate, and megestrol acetate.[21][279]
Research
CPA has been studied and used in combination with low-dose diethylstilbestrol in the treatment of prostate cancer.[280][281] The combination results in suppression of testosterone levels into the castrate range, which normally cannot be achieved with CPA alone.[281] CPA has been studied as a form of androgen deprivation therapy for the treatment of benign prostatic hyperplasia (enlarged prostate).[282][283][284]
CPA has been studied for use as a potential male hormonal contraceptive in combination with testosterone in men.[285] CPA was under development by Barr Pharmaceuticals in the 2000s for the treatment of hot flashes in prostate cancer patients in the United States.[286] It reached phase III clinical trials for this indication and had the tentative brand name CyPat but development was ultimately discontinued in 2008.[286] CPA is not satisfactorily effective as topical antiandrogen, for instance in the treatment of acne.[107]
CPA has been investigated for use in reducing aggression and self-injurious behavior via its antiandrogenic effects in conditions like autism spectrum disorders, dementias like Alzheimer's disease, and psychosis.[287][288][289][290] CPA may be effective in the treatment of obsessive–compulsive disorder (OCD).[291] In very limited clinical research, it has been reported to be "considerably" effective in the treatment of OCD in women.[292][293] CPA has been studied in the treatment of cluster headaches in men.[294]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 3 4 5 6 Huber J, Zeillinger R, Schmidt J, Täuber U, Kuhnz W, Spona J (November 1988). "Pharmacokinetics of cyproterone acetate and its main metabolite 15 beta-hydroxy-cyproterone acetate in young healthy women". Int J Clin Pharmacol Ther Toxicol. 26 (11): 555–61. PMID 2977383.
- 1 2 3 Bińkowska M, Woroń J (June 2015). "Progestogens in menopausal hormone therapy". Przegla̜d Menopauzalny = Menopause Review. 14 (2): 134–43. doi:10.5114/pm.2015.52154. PMC 4498031. PMID 26327902.
- 1 2 Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (December 2003). "Classification and pharmacology of progestins" (PDF). Maturitas. 46 Suppl 1: S7–S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
Since there is no binding of CPA to SHBG and CBG in the serum, 93% of the compound is bound to serum albumin.
- 1 2 Wakelin SH, Maibach HI, Archer CB (1 June 2002). Systemic Drug Treatment in Dermatology: A Handbook. CRC Press. pp. 32–. ISBN 978-1-84076-013-2.
It is almost exclusively bound to plasma albumin.
- 1 2 Hammond GL, Lähteenmäki PL, Lähteenmäki P, Luukkainen T (October 1982). "Distribution and percentages of non-protein bound contraceptive steroids in human serum". Journal of Steroid Biochemistry. 17 (4): 375–80. doi:10.1016/0022-4731(82)90629-X. PMID 6215538.
- 1 2 Dickman A (27 September 2012). Drugs in Palliative Care. OUP Oxford. pp. 137–138. ISBN 978-0-19-966039-1.
- ↑ Boarder M, Newby D, Navti P (25 March 2010). Pharmacology for Pharmacy and the Health Sciences: A Patient-centred Approach. OUP Oxford. pp. 632–. ISBN 978-0-19-955982-4.
- 1 2 3 4 Frith RG, Phillipou G (1985). "15-Hydroxycyproterone acetate and cyproterone acetate levels in plasma and urine". J. Chromatogr. 338 (1): 179–86. doi:10.1016/0378-4347(85)80082-7. PMID 3160716.
- 1 2 3 4 5 6 7 8 Georg F. Weber (22 July 2015). Molecular Therapies of Cancer. Springer. pp. 316–. ISBN 978-3-319-13278-5.
The terminal half-life is about 38 h. A portion of the drug is metabolized by hydrolysis to cyproterone and acetic acid. However, in contrast to many other steroid esters hydrolysis is not extensive, and much of the pharmacological activity is exerted by the acetate form. Excretion is about 70% in the feces, mainly in the form of glucuronidated metabolites, and about 30% in the urine, predominantly as non-conjugated metabolites.
- 1 2 3 4 5 6 7 8 9 Barradell LB, Faulds D (July 1994). "Cyproterone. A review of its pharmacology and therapeutic efficacy in prostate cancer". Drugs Aging. 5 (1): 59–80. doi:10.2165/00002512-199405010-00006. PMID 7919640.
- 1 2 3 AAPL Newsletter (PDF). The Academy. 1998.
CPA is 100% bioavailable when taken orally with a half life of 38 hours. The injectable form reaches maximum plasma levels in 82 hours and has a half life of about 72 hours.
- 1 2 3 4 5 6 7 8 Neumann F (1994). "The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research". Exp. Clin. Endocrinol. 102 (1): 1–32. doi:10.1055/s-0029-1211261. PMID 8005205.
- ↑ Neumann F (January 1977). "Pharmacology and potential use of cyproterone acetate". Horm. Metab. Res. 9 (1): 1–13. doi:10.1055/s-0028-1093574. PMID 66176.
- ↑ Neumann F, Töpert M (November 1986). "Pharmacology of antiandrogens". Journal of Steroid Biochemistry. 25 (5B): 885–95. doi:10.1016/0022-4731(86)90320-1. PMID 2949114.
- ↑ Jonathan S. Berek (2007). Berek & Novak's Gynecology. Lippincott Williams & Wilkins. p. 1085. ISBN 978-0-7817-6805-4.
- 1 2 3 4 5 6 7 8 9 10 Pucci E, Petraglia F (December 1997). "Treatment of androgen excess in females: yesterday, today and tomorrow". Gynecol. Endocrinol. 11 (6): 411–33. doi:10.3109/09513599709152569. PMID 9476091.
- 1 2 Neumann F, Elger W (1966). "Permanent changes in gonadal function and sexual behaviour as a result of early feminization of male rats by treatment with an antiandrogenic steroid". Endokrinologie. 50: 209–225.
- 1 2 3 Sarah H. Wakelin (1 June 2002). Systemic Drug Treatment in Dermatology: A Handbook. CRC Press. p. 32. ISBN 978-1-84076-013-2.
- 1 2 3 4 G. Plewig; A.M. Kligman (6 December 2012). ACNE and ROSACEA. Springer Science & Business Media. pp. 662, 685. ISBN 978-3-642-59715-2.
- 1 2 Louw-du Toit R, Storbeck KH, Cartwright M, Cabral A, Africander D (February 2017). "Progestins used in endocrine therapy and the implications for the biosynthesis and metabolism of endogenous steroid hormones". Mol. Cell. Endocrinol. 441: 31–45. doi:10.1016/j.mce.2016.09.004. PMID 27616670.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 https://www.drugs.com/international/cyproterone.html
- 1 2 3 4 5 6 7 8 9 10 11 12 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 289–. ISBN 978-3-88763-075-1.
- 1 2 Loren S Schechter (22 September 2016). Surgical Management of the Transgender Patient. Elsevier Health Sciences. pp. 26–. ISBN 978-0-323-48408-4.
- 1 2 J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2293, 2464, 2479, 6225. ISBN 978-0-323-32195-2.
- ↑ Mario Maggi (17 November 2011). Hormonal Therapy for Male Sexual Dysfunction. John Wiley & Sons. p. 104. ISBN 978-1-119-96380-6.
- 1 2 Duker M, Malsch M (28 January 2013). Incapacitation: Trends and New Perspectives. Ashgate Publishing, Ltd. p. 77. ISBN 978-1-4094-7151-6.
- ↑ "Mercilon - Summary of Product Characteristics (SmPC) - (eMC)". www.medicines.org.uk. Retrieved 2018-07-12.
- ↑ Fruzzetti F, Trémollieres F, Bitzer J (May 2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- ↑ Fruzzetti F, Bitzer J (2010). "Review of clinical experience with estradiol in combined oral contraceptives". Contraception. 81 (1): 8–15. doi:10.1016/j.contraception.2009.08.010. PMID 20004267.
- 1 2 Van der Spuy ZM, le Roux PA (2003). "Cyproterone acetate for hirsutism". Cochrane Database Syst Rev (4): CD001125. doi:10.1002/14651858.CD001125. PMID 14583927.
- 1 2 3 Bitzer J, Römer T, Lopes da Silva Filho A (June 2017). "The use of cyproterone acetate/ethinyl estradiol in hyperandrogenic skin symptoms - a review". Eur J Contracept Reprod Health Care. 22 (3): 172–182. doi:10.1080/13625187.2017.1317339. PMID 28447864.
- 1 2 Beylot C, Doutre MS, Beylot-Barry M (1998). "Oral contraceptives and cyproterone acetate in female acne treatment". Dermatology (Basel). 196 (1): 148–52. doi:10.1159/000017849. PMID 9557250.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Miller JA, Jacobs HS (May 1986). "Treatment of hirsutism and acne with cyproterone acetate". Clin Endocrinol Metab. 15 (2): 373–89. doi:10.1016/S0300-595X(86)80031-7. PMID 2941191.
- ↑ Ingram JR, Woo PN, Chua SL, Ormerod AD, Desai N, Kai AC, Hood K, Burton T, Kerdel F, Garner SE, Piguet V (October 2015). "Interventions for hidradenitis suppurativa". Cochrane Database Syst Rev (10): CD010081. doi:10.1002/14651858.CD010081.pub2. PMID 26443004.
- ↑ Ioannides D, Lazaridou E (2015). "Female pattern hair loss". Curr. Probl. Dermatol. 47: 45–54. doi:10.1159/000369404. PMID 26370643.
- 1 2 Wiegratz I, Kuhl H (2002). "Managing cutaneous manifestations of hyperandrogenic disorders: the role of oral contraceptives". Treat Endocrinol. 1 (6): 372–86. doi:10.2165/00024677-200201060-00003. PMID 15832490.
- ↑ Arowojolu AO, Gallo MF, Lopez LM, Grimes DA (July 2012). "Combined oral contraceptive pills for treatment of acne". Cochrane Database Syst Rev (7): CD004425. doi:10.1002/14651858.CD004425.pub6. PMID 22786490.
- 1 2 3 4 5 6 Bentham Science Publishers (September 1999). Current Pharmaceutical Design. Bentham Science Publishers. pp. 712, 716–717, 1110.
- ↑ Ruan X, Kubba A, Aguilar A, Mueck AO (June 2017). "Use of cyproterone acetate/ethinylestradiol in polycystic ovary syndrome: rationale and practical aspects". Eur J Contracept Reprod Health Care. 22 (3): 183–190. doi:10.1080/13625187.2017.1317735. PMID 28463030.
- 1 2 3 4 Laron Z, Kauli R (July 2000). "Experience with cyproterone acetate in the treatment of precocious puberty". J. Pediatr. Endocrinol. Metab. 13 Suppl 1: 805–10. doi:10.1515/JPEM.2000.13.S1.805. PMID 10969925.
- ↑ Catteau-Jonard S, Cortet-Rudelli C, Richard-Proust C, Dewailly D (2012). "Hyperandrogenism in adolescent girls". Endocr Dev. 22: 181–93. doi:10.1159/000326688. PMID 22846529.
- ↑ Reismann P, Likó I, Igaz P, Patócs A, Rácz K (August 2009). "Pharmacological options for treatment of hyperandrogenic disorders". Mini Rev Med Chem. 9 (9): 1113–26. doi:10.2174/138955709788922692. PMID 19689407.
- ↑ Gooren LJ, Giltay EJ, Bunck MC (January 2008). "Long-term treatment of transsexuals with cross-sex hormones: extensive personal experience". J. Clin. Endocrinol. Metab. 93 (1): 19–25. doi:10.1210/jc.2007-1809. PMID 17986639.
- ↑ Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG (November 2017). "Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline". J. Clin. Endocrinol. Metab. 102 (11): 3869–3903. doi:10.1210/jc.2017-01658. PMID 28945902.
- 1 2 Chew D, Anderson J, Williams K, May T, Pang K (April 2018). "Hormonal Treatment in Young People With Gender Dysphoria: A Systematic Review". Pediatrics. 141 (4). doi:10.1542/peds.2017-3742. PMID 29514975.
- ↑ Deutsch M (17 June 2016), Guidelines for the Primary and Gender-Affirming Care of Transgender and Gender Nonbinary People (PDF) (2nd ed.), University of California, San Francisco: Center of Excellence for Transgender Health, p. 28
- ↑ James Barrett (29 September 2017). Transsexual and Other Disorders of Gender Identity: A Practical Guide to Management. CRC Press. pp. 216–217, 221. ISBN 978-1-315-34513-0.
- ↑ Randi Ettner; Stan Monstrey; Eli Coleman (20 May 2016). Principles of Transgender Medicine and Surgery. Routledge. pp. 169, 171, 216. ISBN 978-1-317-51460-2.
- ↑ Gianna E. Israel (March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 66–. ISBN 978-1-56639-852-7.
- ↑ Panagiotakopoulos, Leonidas (2018). "Transgender medicine - puberty suppression". Reviews in Endocrine and Metabolic Disorders. doi:10.1007/s11154-018-9457-0. ISSN 1389-9155.
- ↑ Rosenthal SM (December 2014). "Approach to the patient: transgender youth: endocrine considerations". J. Clin. Endocrinol. Metab. 99 (12): 4379–89. doi:10.1210/jc.2014-1919. PMID 25140398.
- ↑ Mahfouda S, Moore JK, Siafarikas A, Zepf FD, Lin A (October 2017). "Puberty suppression in transgender children and adolescents". Lancet Diabetes Endocrinol. 5 (10): 816–826. doi:10.1016/S2213-8587(17)30099-2. PMID 28546095.
- ↑ Tack LJ, Heyse R, Craen M, Dhondt K, Bossche H, Laridaen J, Cools M (May 2017). "Consecutive Cyproterone Acetate and Estradiol Treatment in Late-Pubertal Transgender Female Adolescents". J Sex Med. 14 (5): 747–757. doi:10.1016/j.jsxm.2017.03.251. PMID 28499525.
- 1 2 3 Torri V, Floriani I (June 2005). "Cyproterone acetate in the therapy of prostate carcinoma". Arch Ital Urol Androl. 77 (3): 157–63. PMID 16372511.
- ↑ Schröder, Fritz H. (1996). "Cyproterone Acetate — Results of Clinical Trials and Indications for Use in Human Prostate Cancer": 45–51. doi:10.1007/978-3-642-45745-6_4.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Schröder FH (December 1993). "Cyproterone acetate--mechanism of action and clinical effectiveness in prostate cancer treatment". Cancer. 72 (12 Suppl): 3810–5. doi:10.1002/1097-0142(19931215)72:12+<3810::AID-CNCR2820721710>3.0.CO;2-O. PMID 8252496.
- ↑ de Voogt HJ (1992). "The position of cyproterone acetate (CPA), a steroidal anti-androgen, in the treatment of prostate cancer". Prostate Suppl. 4: 91–5. doi:10.1002/pros.2990210514. PMID 1533452.
- ↑ Goldenberg SL, Bruchovsky N (February 1991). "Use of cyproterone acetate in prostate cancer". Urol. Clin. North Am. 18 (1): 111–22. PMID 1825143.
- ↑ Thomas L. Lemke; David A. Williams (2008). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1288–. ISBN 978-0-7817-6879-5.
- 1 2 3 Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, Wilt TJ (April 2000). "Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis". Ann. Intern. Med. 132 (7): 566–77. doi:10.7326/0003-4819-132-7-200004040-00009. PMID 10744594.
- 1 2 3 Mydlo JH, Godec CJ (29 September 2015). Prostate Cancer: Science and Clinical Practice. Elsevier Science. pp. 516–521, 534–540. ISBN 978-0-12-800592-7. Archived from the original on 8 September 2017.
- ↑ Miyamoto H, Messing EM, Chang C (2004). "Androgen deprivation therapy for prostate cancer: current status and future prospects". The Prostate. 61 (4): 332–53. doi:10.1002/pros.20115. PMID 15389811.
- ↑ Wirth MP, Hakenberg OW, Froehner M (February 2007). "Antiandrogens in the treatment of prostate cancer". European Urology. 51 (2): 306–13, discussion 314. doi:10.1016/j.eururo.2006.08.043. PMID 17007995.
- 1 2 3 4 Singh, Shankar; Gauthier, Sylvain; Labrie, Fernand (2000). "Androgen Receptor Antagonists (Antiandrogens) Structure-Activity Relationships". Current Medicinal Chemistry. 7 (2): 211–247. doi:10.2174/0929867003375371. ISSN 0929-8673. PMID 10637363.
When compared to flutamide, [cyproterone acetate] has significant intrinsic androgenic and estrogenic activities. [...] The effects of flutamide and the steroidal derivatives, cyproterone acetate, chlormadinone acetate, megestrol acetate and medroxyprogesterone acetate were compared in vivo in female nude mice bearing androgen-sensitive Shionogi tumors. All steroidal compounds stimulated tumor growth while flutamide had no stimulatory effect [51]. Thus, CPA due to its intrinsic properties stimulates androgen-sensitive parameters and cancer growth. Cyproterone acetate added to castration has never been shown in any controlled study to prolong disease-free survival or overall survival in prostate cancer when compared with castration alone [152-155].
- ↑ Kaliks RA, Del Giglio A (2008). "Management of advanced prostate cancer" (PDF). Revista Da Associação Médica Brasileira. 54 (2): 178–82. doi:10.1590/S0104-42302008000200025. PMID 18506331. Archived (PDF) from the original on 10 May 2017.
- ↑ Chabner BA, Longo DL (8 November 2010). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott Williams & Wilkins. pp. 679–680. ISBN 978-1-60547-431-1.
From a structural standpoint, antiandrogens are classified as steroidal, including cyproterone [acetate] (Androcur) and megestrol [acetate], or nonsteroidal, including flutamide (Eulexin, others), bicalutamide (Casodex), and nilutamide (Nilandron). The steroidal antiandrogens are rarely used.
- ↑ Alan Horwich (11 February 2010). Systemic Treatment of Prostate Cancer. OUP Oxford. pp. 44–. ISBN 978-0-19-956142-1.
- 1 2 Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU Int. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397.
- 1 2 Dalesio O, van Tinteren H, Clarke M, Peto R, Schroder F, Dechering I, Evans V, Godwin J, Blumenstein B, Crawford E, Denis L, Hall R, Hill C, Iversen P, Shipley W, Soloway M, Sylvester R (2000). "Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials". The Lancet. 355 (9214): 1491–1498. doi:10.1016/S0140-6736(00)02163-2. ISSN 0140-6736.
- ↑ Louis Denis (6 December 2012). Antiandrogens in Prostate Cancer: A Key to Tailored Endocrine Treatment. Springer Science & Business Media. pp. 70–. ISBN 978-3-642-45745-6.
- 1 2 3 Chodak G, Gomella L, Phung de H (September 2007). "Combined androgen blockade in advanced prostate cancer: looking back to move forward". Clin Genitourin Cancer. 5 (6): 371–8. doi:10.3816/CGC.2007.n.019. PMID 17956709.
- ↑ Khan O, Ferriter M, Huband N, Powney MJ, Dennis JA, Duggan C (February 2015). "Pharmacological interventions for those who have sexually offended or are at risk of offending". Cochrane Database Syst Rev (2): CD007989. doi:10.1002/14651858.CD007989.pub2. PMID 25692326.
- 1 2 Silvani M, Mondaini N, Zucchi A (September 2015). "Androgen deprivation therapy (castration therapy) and pedophilia: What's new". Arch Ital Urol Androl. 87 (3): 222–6. doi:10.4081/aiua.2015.3.222. PMID 26428645.
- ↑ Reilly DR, Delva NJ, Hudson RW (August 2000). "Protocols for the use of cyproterone, medroxyprogesterone, and leuprolide in the treatment of paraphilia". Can J Psychiatry. 45 (6): 559–63. doi:10.1177/070674370004500608. PMID 10986575.
- 1 2 3 Neumann F, Kalmus J (1991). "Cyproterone acetate in the treatment of sexual disorders: pharmacological base and clinical experience". Exp. Clin. Endocrinol. 98 (2): 71–80. doi:10.1055/s-0029-1211103. PMID 1838080.
- ↑ Freund K (1980). "Therapeutic sex drive reduction". Acta Psychiatr Scand Suppl. 287: 5–38. doi:10.1111/j.1600-0447.1980.tb10433.x. PMID 7006321.
- ↑ Codispoti VL (December 2008). "Pharmacology of sexually compulsive behavior". Psychiatr. Clin. North Am. 31 (4): 671–9. doi:10.1016/j.psc.2008.06.002. PMID 18996306.
- 1 2 3 Assumpção AA, Garcia FD, Garcia HD, Bradford JM, Thibaut F (June 2014). "Pharmacologic treatment of paraphilias". Psychiatr. Clin. North Am. 37 (2): 173–81. doi:10.1016/j.psc.2014.03.002. PMID 24877704.
- 1 2 3 Guay DR (December 2008). "Inappropriate sexual behaviors in cognitively impaired older individuals". Am J Geriatr Pharmacother. 6 (5): 269–88. doi:10.1016/j.amjopharm.2008.12.004. PMID 19161930.
- ↑ Clarke DJ (April 1989). "Antilibidinal drugs and mental retardation: a review". Med Sci Law. 29 (2): 136–46. doi:10.1177/002580248902900209. PMID 2526280.
- ↑ Hucker SJ (June 1985). "Self-harmful sexual behavior". Psychiatr. Clin. North Am. 8 (2): 323–37. PMID 3895195.
- 1 2 3 4 5 6 Laschet U, Laschet L (June 1975). "Antiandrogens in the treatment of sexual deviations of men". J. Steroid Biochem. 6 (6): 821–6. doi:10.1016/0022-4731(75)90310-6. PMID 1177426.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 Neumann, Friedmund (1996). "Pharmacology of Cyproterone Acetate — A Short Review": 31–44. doi:10.1007/978-3-642-45745-6_3.
- ↑ Brito VN, Latronico AC, Arnhold IJ, Mendonça BB (February 2008). "Update on the etiology, diagnosis and therapeutic management of sexual precocity". Arq Bras Endocrinol Metabol. 52 (1): 18–31. doi:10.1590/S0004-27302008000100005. PMID 18345393.
- ↑ Anupam Sachdeva; AK Dutta (31 August 2012). Advances in Pediatrics. JP Medical Ltd. pp. 1202–. ISBN 978-93-5025-777-7.
- ↑ Holland FJ (March 1991). "Gonadotropin-independent precocious puberty". Endocrinol. Metab. Clin. North Am. 20 (1): 191–210. PMID 1903104.
- 1 2 Schneider HP (December 2000). "The role of antiandrogens in hormone replacement therapy". Climacteric. 3 Suppl 2: 21–7. PMID 11379383.
- ↑ Frisk J (January 2010). "Managing hot flushes in men after prostate cancer--a systematic review". Maturitas. 65 (1): 15–22. doi:10.1016/j.maturitas.2009.10.017. PMID 19962840.
- ↑ Spetz AC, Zetterlund EL, Varenhorst E, Hammar M (2003). "Incidence and management of hot flashes in prostate cancer". J Support Oncol. 1 (4): 263–6, 269–70, 272–3, discussion 267–8, 271–2. PMID 15334868.
- ↑ Radlmaier A, Bormacher K, Neumann F (1990). "Hot flushes: mechanism and prevention". Prog. Clin. Biol. Res. 359: 131–40, discussion 141–53. PMID 2149457.
- ↑ Louis J Denis; Keith Griffiths; Amir V Kaisary; Gerald P Murphy (1 March 1999). Textbook of Prostate Cancer: Pathology, Diagnosis and Treatment: Pathology, Diagnosis and Treatment. CRC Press. pp. 308–. ISBN 978-1-85317-422-3.
- ↑ Sugiono M, Winkler MH, Okeke AA, Benney M, Gillatt DA (2005). "Bicalutamide vs cyproterone acetate in preventing flare with LHRH analogue therapy for prostate cancer—a pilot study". Prostate Cancer and Prostatic Diseases. 8 (1): 91–4. doi:10.1038/sj.pcan.4500784. PMID 15711607.
- ↑ Bruchovsky N, Goldenberg SL, Akakura K, Rennie PS (September 1993). "Luteinizing hormone-releasing hormone agonists in prostate cancer. Elimination of flare reaction by pretreatment with cyproterone acetate and low-dose diethylstilbestrol". Cancer. 72 (5): 1685–91. doi:10.1002/1097-0142(19930901)72:5<1685::AID-CNCR2820720532>3.0.CO;2-3. PMID 7688656.
- 1 2 Jürgen Engel; Axel Kleemann; Bernhard Kutscher; Dietmar Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 351–352. ISBN 978-3-13-179275-4.
- ↑ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 79–. ISBN 978-3-7692-2114-5.
- 1 2 Jean L. Bolognia; Joseph L. Jorizzo; Julie V. Schaffer (8 June 2012). Dermatology E-Book. Elsevier Health Sciences. pp. 557–. ISBN 0-7020-5182-9.
- ↑ Peter Altmeyer (2 July 2013). Therapielexikon Dermatologie und Allergologie. Springer-Verlag. pp. 27–. ISBN 978-3-662-10498-9.
- ↑ Jerome P. Kassirer; Harry L. Greene (1997). Current Therapy in Adult Medicine. Mosby. p. 174. ISBN 978-0-8151-5480-8.
- ↑ "Cyproterone acetate - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2018-07-11.
- ↑ Mohan D, Taylor R, Mackeith JA (1998). "Cyproterone acetate and striae". International Journal of Psychiatry in Clinical Practice. 2 (2): 147–8. doi:10.3109/13651509809115348. PMID 24946296.
- ↑ Bachelot A, Chabbert-Buffet N, Salenave S, Kerlan V, Galand-Portier MB (February 2010). "Anti-androgen treatments". Ann. Endocrinol. (Paris). 71 (1): 19–24. doi:10.1016/j.ando.2009.12.001. PMID 20096826.
- 1 2 Guay DR (January 2009). "Drug treatment of paraphilic and nonparaphilic sexual disorders". Clin Ther. 31 (1): 1–31. doi:10.1016/j.clinthera.2009.01.009. PMID 19243704.
No quantitative data on these adverse events are available, even in the product prescribing information and data sheets.
- ↑ https://www.medicines.org.uk/emc/files/pil.1120.pdf
- ↑ http://www.bayerresources.com.au/resources/uploads/PI/file9701.pdf
- 1 2 3 4 Heinemann LA, Will-Shahab L, van Kesteren P, Gooren LJ (May 1997). "Safety of cyproterone acetate: report of active surveillance". Pharmacoepidemiol Drug Saf. 6 (3): 169–78. doi:10.1002/(SICI)1099-1557(199705)6:3<169::AID-PDS263>3.0.CO;2-3. PMID 15073785.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Hughes A, Hasan SH, Oerter GW, Voss HE, Banner F, Neumann F, et al. (27 November 2013). Androgens II and Antiandrogens / Androgene II und Antiandrogene. Springer Science & Business Media. pp. 474, 485, 489–492. ISBN 978-3-642-80859-3.
- 1 2 Iversen P, Melezinek I, Schmidt A (January 2001). "Nonsteroidal antiandrogens: a therapeutic option for patients with advanced prostate cancer who wish to retain sexual interest and function". BJU International. 87 (1): 47–56. doi:10.1046/j.1464-410x.2001.00988.x. PMID 11121992.
- ↑ Terrence Priestman (26 May 2012). Cancer Chemotherapy in Clinical Practice. Springer Science & Business Media. pp. 97–. ISBN 978-0-85729-727-3.
- ↑ Di Lorenzo G, Autorino R, Perdonà S, De Placido S (2005). "Management of gynaecomastia in patients with prostate cancer: a systematic review". Lancet Oncol. 6 (12): 972–9. doi:10.1016/S1470-2045(05)70464-2. PMID 16321765.
- ↑ Hirawat S, Budman DR, Kreis W (June 2003). "The androgen receptor: structure, mutations, and antiandrogens". Cancer Invest. 21 (3): 400–17. doi:10.1081/CNV-120018232. PMID 12901287.
- ↑ Blume-Peytavi U, Whiting DA, Trüeb RM (26 June 2008). Hair Growth and Disorders. Springer Science & Business Media. pp. 181–. ISBN 978-3-540-46911-7.
"Potential side-effects are dose related and include menstrual irregularity, weight gain, breast tenderness, decreased libido, depression, and nausea.
- ↑ James Barrett (2007). Transsexual and Other Disorders of Gender Identity: A Practical Guide to Management. Radcliffe Publishing. p. 174. ISBN 978-1-85775-719-4.
- ↑ Barth JH, Cherry CA, Wojnarowska F, Dawber RP (July 1991). "Cyproterone acetate for severe hirsutism: results of a double-blind dose-ranging study". Clinical Endocrinology. 35 (1): 5–10. doi:10.1111/j.1365-2265.1991.tb03489.x. PMID 1832346.
- 1 2 3 Rushton DH (July 2002). "Nutritional factors and hair loss". Clinical and Experimental Dermatology. 27 (5): 396–404. doi:10.1046/j.1365-2230.2002.01076.x. PMID 12190640.
- ↑ Seal LJ, Franklin S, Richards C, Shishkareva A, Sinclaire C, Barrett J (December 2012). "Predictive markers for mammoplasty and a comparison of side effect profiles in transwomen taking various hormonal regimens". The Journal of Clinical Endocrinology and Metabolism. 97 (12): 442–8. doi:10.1210/jc.2012-2030. PMID 23055547.
- ↑ Boccardo F (August 2000). "Hormone therapy of prostate cancer: is there a role for antiandrogen monotherapy?". Critical Reviews in Oncology/Hematology. 35 (2): 121–32. doi:10.1016/S1040-8428(00)00051-2. PMID 10936469.
- ↑ Clive Peedell (2005). Concise Clinical Oncology. Elsevier Health Sciences. pp. 81–. ISBN 0-7506-8836-X.
- ↑ Damber JE (2005). "Endocrine therapy for prostate cancer". Acta Oncologica. 44 (6): 605–9. doi:10.1080/02841860510029743. PMID 16165920.
- ↑ Robert G. Lahita (9 June 2004). Systemic Lupus Erythematosus. Academic Press. pp. 797–. ISBN 978-0-08-047454-0.
- 1 2 Waken SH, Maibach HI, Archer CB (21 May 2015). Handbook of Systemic Drug Treatment in Dermatology (Second ed.). CRC Press. pp. 34–. ISBN 978-1-4822-2286-9.
- 1 2 Ralph M. Trüeb (26 February 2013). Female Alopecia: Guide to Successful Management. Springer Science & Business Media. pp. 46–. ISBN 978-3-642-35503-5.
- 1 2 Ramsay ID, Rushton DH (July 1990). "Reduced serum vitamin B12 levels during oral cyproterone-acetate and ethinyl-oestradiol therapy in women with diffuse androgen-dependent alopecia". Clinical and Experimental Dermatology. 15 (4): 277–81. doi:10.1111/j.1365-2230.1990.tb02089.x. PMID 2145099.
- ↑ Sadock BJ, Sadock VA (2010). Kaplan and Sadock's Pocket Handbook of Clinical Psychiatry. Lippincott Williams & Wilkins. pp. 582–. ISBN 978-1-60547-264-5.
- ↑ Musisi S, Jacobson S (14 April 2015). Brain Degeneration and Dementia in Sub-Saharan Africa. Springer. pp. 60–. ISBN 978-1-4939-2456-1.
- ↑ Müller E (18 September 2003). Peptides and Non Peptides of Oncologic and Neuroendocrine Relevance: From Basic to Clinical Research. Springer Science & Business Media. pp. 171–. ISBN 978-88-470-0295-1. Archived from the original on 8 September 2017.
[CPA] induces relevant effects on the coagulative system. A recent meta-analysis relating to total androgenic blockade has shown that cyproterone acetate when combined with castration reduces the long-term efficacy of androgen-suppressive treatments. In fact, it causes an increase in treatment-related mortality, mainly due to cardiovascular complications (No authors, 2000).
- 1 2 Furr BJ, Tucker H (January 1996). "The preclinical development of bicalutamide: pharmacodynamics and mechanism of action". Urology. 47 (1A Suppl): 13–25, discussion 29–32. doi:10.1016/S0090-4295(96)80003-3. PMID 8560673.
- ↑ Migliari R, Muscas G, Murru M, Verdacchi T, De Benedetto G, De Angelis M (1999). "Antiandrogens: a summary review of pharmacodynamic properties and tolerability in prostate cancer therapy". Archivio Italiano di Urologia e Andrologia. 71 (5): 293–302. PMID 10673793.
The only advantage of cyproterone acetate on pure antiandrogens seems to be the low incidence of hot flushes; [...] However, hepatotoxicity associated with long term daily doses of 300 mg daily and the unacceptably high incidence of cardiovascular side effects (10%) should restrict its use to patients who are intolerant of pure antiandrogen compound. In contrast to steroidal compound nonsteroidal compounds let sexual potency to be retained, [...]
- ↑ Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clinical Pharmacokinetics. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622.
- 1 2 Newling DW (March 1997). "The palliative therapy of advanced prostate cancer, with particular reference to the results of recent European clinical trials". Br J Urol. 79 Suppl 1: 72–81. doi:10.1111/j.1464-410X.1997.tb00805.x. PMID 9088277.
- ↑ Mahler C, Verhelst J, Denis L (May 1998). "Clinical pharmacokinetics of the antiandrogens and their efficacy in prostate cancer". Clin Pharmacokinet. 34 (5): 405–17. doi:10.2165/00003088-199834050-00005. PMID 9592622.
- ↑ Anderson J (March 2003). "The role of antiandrogen monotherapy in the treatment of prostate cancer". BJU International. 91 (5): 455–61. doi:10.1046/j.1464-410X.2003.04026.x. PMID 12603397.
- 1 2 Aronson JK (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 150–152. ISBN 978-0-08-093292-7.
- ↑ Chang C (22 March 2005). Prostate Cancer: Basic Mechanisms and Therapeutic Approaches. World Scientific. pp. 10–11. ISBN 978-981-4481-61-8. Archived from the original on 8 September 2017.
- 1 2 3 4 Neil Kaplowitz (16 October 2002). Drug-Induced Liver Disease. CRC Press. pp. 618–. ISBN 978-0-203-90912-6.
- 1 2 3 Kim JH, Yoo BW, Yang WJ (May 2014). "Hepatic failure induced by cyproterone acetate: A case report and literature review". Canadian Urological Association Journal = Journal De l'Association Des Urologues Du Canada. 8 (5–6): E458–61. doi:10.5489/cuaj.1753. PMC 4081269. PMID 25024808.
- 1 2 3 4 5 6 7 8 Savidou I, Deutsch M, Soultati AS, Koudouras D, Kafiri G, Dourakis SP (December 2006). "Hepatotoxicity induced by cyproterone acetate: a report of three cases". World Journal of Gastroenterology. 12 (46): 7551–5. doi:10.3748/wjg.v12.i46.7551. PMC 4087608. PMID 17167851.
- ↑ Hinkel A, Berges RR, Pannek J, Schulze H, Senge T (1996). "Cyproterone acetate in the treatment of advanced prostatic cancer: retrospective analysis of liver toxicity in the long-term follow-up of 89 patients". Eur. Urol. 30 (4): 464–70. doi:10.1159/000474216. PMID 8977068.
- 1 2 Gava, Giulia; Seracchioli, Renato; Meriggiola, Maria Cristina (2017). "Therapy with Antiandrogens in Gender Dysphoric Natal Males". Endocrinology of the Testis and Male Reproduction. Endocrinology. pp. 1199–1209. doi:10.1007/978-3-319-44441-3_42. ISBN 978-3-319-44440-6. ISSN 2510-1927.
- ↑ Marius Duker (23 May 2016). Incapacitation: Trends and New Perspectives. Routledge. pp. 139–. ISBN 978-1-317-11767-4.
- 1 2 Berlex Canada, Inc. (2003-02-10). "Cyproterone Acetate Tablets and Injections Product Monographs (revised version)" (PDF). Archived from the original (PDF) on 2006-09-24.
- ↑ Adverse Drug Reactions Advisory Committee (February 2004). "Australian Adverse Drug Reactions Bulletin, Volume 23, Number 1".
- 1 2 Kromm J, Jeerakathil T (June 2014). "Cyproterone acetate-ethinyl estradiol use in a 23-year-old woman with stroke". CMAJ. 186 (9): 690–3. doi:10.1503/cmaj.130579. PMC 4049993. PMID 24491473.
- ↑ Vasilakis-Scaramozza C, Jick H (October 2001). "Risk of venous thromboembolism with cyproterone or levonorgestrel contraceptives". Lancet. 358 (9291): 1427–9. doi:10.1016/S0140-6736(01)06522-9. PMID 11705493.
- ↑ Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E (October 2011). "Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9". BMJ. 343: d6423. doi:10.1136/bmj.d6423. PMC 3202015. PMID 22027398.
- ↑ Wiegratz I, Kuhl H (September 2006). "Metabolic and clinical effects of progestogens". Eur J Contracept Reprod Health Care. 11 (3): 153–61. doi:10.1080/13625180600772741. PMID 17056444.
- 1 2 3 4 5 6 7 8 9 10 Asscheman H, T'Sjoen G, Lemaire A, Mas M, Meriggiola MC, Mueller A, Kuhn A, Dhejne C, Morel-Journel N, Gooren LJ (September 2014). "Venous thrombo-embolism as a complication of cross-sex hormone treatment of male-to-female transsexual subjects: a review". Andrologia. 46 (7): 791–5. doi:10.1111/and.12150. PMID 23944849.
- 1 2 3 Beyer-Westendorf J, Werth S, Halbritter K, Weiss N (April 2010). "Cancer in males and risk of venous thromboembolism". Thromb. Res. 125 Suppl 2: S155–9. doi:10.1016/S0049-3848(10)70035-9. PMID 20433997.
- 1 2 3 4 Seaman HE, Langley SE, Farmer RD, de Vries CS (June 2007). "Venous thromboembolism and cyproterone acetate in men with prostate cancer: a study using the General Practice Research Database". BJU Int. 99 (6): 1398–403. doi:10.1111/j.1464-410X.2007.06859.x. PMID 17537215.
- ↑ Van Hemelrijck M, Adolfsson J, Garmo H, Bill-Axelson A, Bratt O, Ingelsson E, Lambe M, Stattin P, Holmberg L (May 2010). "Risk of thromboembolic diseases in men with prostate cancer: results from the population-based PCBaSe Sweden". Lancet Oncol. 11 (5): 450–8. doi:10.1016/S1470-2045(10)70038-3. PMC 2861771. PMID 20395174.
- 1 2 Raj R, Korja M, Koroknay-Pál P, Niemelä M (2018). "Multiple meningiomas in two male-to-female transsexual patients with hormone replacement therapy: A report of two cases and a brief literature review". Surg Neurol Int. 9: 109. doi:10.4103/sni.sni_22_18. PMC 5991277. PMID 29930875.
- ↑ Nota NM, Wiepjes CM, de Blok CJ, Gooren LJ, Peerdeman SM, Kreukels BP, den Heijer M (July 2018). "The occurrence of benign brain tumours in transgender individuals during cross-sex hormone treatment". Brain. 141 (7): 2047–2054. doi:10.1093/brain/awy108. PMID 29688280.
- 1 2 Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 544–. ISBN 978-3-642-60107-1.
- 1 2 Gooren LJ, Harmsen-Louman W, van Kessel H (February 1985). "Follow-up of prolactin levels in long-term oestrogen-treated male-to-female transsexuals with regard to prolactinoma induction". Clin. Endocrinol. (Oxf). 22 (2): 201–7. doi:10.1111/j.1365-2265.1985.tb01081.x. PMID 3157511.
- ↑ Meningeal Neoplasms—Advances in Research and Treatment: 2012 Edition: ScholarlyBrief. ScholarlyEditions. 26 December 2012. pp. 99–. ISBN 978-1-4816-0002-6.
- ↑ Davey DA (March 2018). "Menopausal hormone therapy: a better and safer future". Climacteric: 1–8. doi:10.1080/13697137.2018.1439915. PMID 29526116.
- ↑ Stanczyk FZ, Bhavnani BR (July 2014). "Use of medroxyprogesterone acetate for hormone therapy in postmenopausal women: is it safe?". J. Steroid Biochem. Mol. Biol. 142: 30–8. doi:10.1016/j.jsbmb.2013.11.011. PMID 24291402.
- 1 2 Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- ↑ Ahmadi H, Daneshmand S (2014). "Androgen deprivation therapy for prostate cancer: long-term safety and patient outcomes". Patient Relat Outcome Meas. 5: 63–70. doi:10.2147/PROM.S52788. PMC 4094624. PMID 25045284.
- ↑ Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- ↑ Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". J. Urol. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- ↑ Warner Chilcott (March 2005). "ESTRACE TABLETS, (estradiol tablets, USP)" (PDF). fda.gov. Retrieved 27 November 2016.
- ↑ Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J (July 2002). "Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial". JAMA. 288 (3): 321–33. doi:10.1001/jama.288.3.321. PMID 12117397.
- ↑ van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, Thijssen JH (April 1990). "Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone". Contraception. 41 (4): 345–52. doi:10.1016/0010-7824(90)90034-S. PMID 2139843.
- ↑ Stalvey JR (July 2002). "Inhibition of 3beta-hydroxysteroid dehydrogenase-isomerase in mouse adrenal cells: a direct effect of testosterone". Steroids. 67 (8): 721–31. doi:10.1016/S0039-128X(02)00023-5. PMID 12117620.
- 1 2 3 4 5 6 7 8 https://pdf.hres.ca/dpd_pm/00011374.PDF
- 1 2 Fabian M. Saleh; Albert J. Grudzinskas; John M. Bradford (11 February 2009). Sex Offenders: Identification, Risk Assessment, Treatment, and Legal Issues. Oxford University Press, USA. pp. 197–. ISBN 978-0-19-517704-6.
- 1 2 3 4 Fritz MA, Speroff L (2011). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 561–. ISBN 978-0-7817-7968-5.
- 1 2 3 4 5 6 Figg W, Chau CH, Small EJ (14 September 2010). Drug Management of Prostate Cancer. Springer. p. 71. ISBN 978-1-60327-829-4.
- 1 2 Honer C, Nam K, Fink C, Marshall P, Ksander G, Chatelain RE, Cornell W, Steele R, Schweitzer R, Schumacher C (May 2003). "Glucocorticoid receptor antagonism by cyproterone acetate and RU486". Molecular Pharmacology. 63 (5): 1012–20. doi:10.1124/mol.63.5.1012. PMID 12695529.
- ↑ Ayub M, Levell MJ (July 1987). "Inhibition of rat testicular 17 alpha-hydroxylase and 17,20-lyase activities by anti-androgens (flutamide, hydroxyflutamide, RU23908, cyproterone acetate) in vitro". Journal of Steroid Biochemistry. 28 (1): 43–7. doi:10.1016/0022-4731(87)90122-1. PMID 2956461.
- ↑ Han C, Davis CB, Wang B (6 January 2010). Evaluation of Drug Candidates for Preclinical Development: Pharmacokinetics, Metabolism, Pharmaceutics, and Toxicology. John Wiley & Sons. pp. 92–. ISBN 978-0-470-57488-1.
- ↑ Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (September 1998). "The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions". The Journal of Clinical Investigation. 102 (5): 1016–23. doi:10.1172/JCI3703. PMC 508967. PMID 9727070.
- ↑ Christians U, Schmitz V, Haschke M (December 2005). "Functional interactions between P-glycoprotein and CYP3A in drug metabolism". Expert Opinion on Drug Metabolism & Toxicology. 1 (4): 641–54. doi:10.1517/17425255.1.4.641. PMID 16863430.
- ↑ Ayub M, Levell MJ (August 1989). "The effect of ketoconazole related imidazole drugs and antiandrogens on [3H] R 1881 binding to the prostatic androgen receptor and [3H]5 alpha-dihydrotestosterone and [3H]cortisol binding to plasma proteins". J. Steroid Biochem. 33 (2): 251–5. doi:10.1016/0022-4731(89)90301-4. PMID 2788775.
- ↑ William B. Pratt (1994). The Anticancer Drugs. Oxford University Press. pp. 219–. ISBN 978-0-19-506739-2.
- 1 2 3 4 5 Kuhl H (2011). "Pharmacology of Progestogens" (PDF). J Reproduktionsmed Endokrinol. 8 (1): 157–177.
- ↑ Schneider HP (November 2003). "Androgens and antiandrogens". Ann. N. Y. Acad. Sci. 997: 292–306. doi:10.1196/annals.1290.033. PMID 14644837.
- 1 2 3 4 Pharmacology of the Skin II: Methods, Absorption, Metabolism and Toxicity, Drugs and Diseases. Springer Science & Business Media. 6 December 2012. pp. 474, 489. ISBN 978-3-642-74054-1.
- 1 2 Sitruk-Ware R (April 2004). "Pharmacological profile of progestins". Maturitas. 47 (4): 277–83. doi:10.1016/j.maturitas.2004.01.001. PMID 15063480.
- ↑ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1152–. ISBN 978-0-7817-1750-2.
- ↑ Kuhl H (April 2004). "Mechanisms of sex steroids. Future developments". Maturitas. 47 (4): 285–91. doi:10.1016/j.maturitas.2003.11.010. PMID 15063481.
- ↑ Simental JA, Sar M, Wilson EM (September 1992). "Domain functions of the androgen receptor". J. Steroid Biochem. Mol. Biol. 43 (1–3): 37–41. PMID 1525065.
- 1 2 Luthy IA, Begin DJ, Labrie F (November 1988). "Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture". Journal of Steroid Biochemistry. 31 (5): 845–52. doi:10.1016/0022-4731(88)90295-6. PMID 2462135.
- 1 2 Térouanne B, Tahiri B, Georget V, Belon C, Poujol N, Avances C, Orio F, Balaguer P, Sultan C (February 2000). "A stable prostatic bioluminescent cell line to investigate androgen and antiandrogen effects". Molecular and Cellular Endocrinology. 160 (1–2): 39–49. doi:10.1016/s0303-7207(99)00251-8. PMID 10715537.
- ↑ Fritz MA, Speroff L (20 December 2010). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. p. 80. ISBN 978-0-7817-7968-5. Retrieved 27 May 2012.
- ↑ Lewis J. Kampel (20 March 2012). Dx/Rx: Prostate Cancer: Prostate Cancer. Jones & Bartlett Publishers. p. 169. ISBN 978-1-4496-8695-6.
- ↑ Prostate Cancer Research Institute. "The Anti-Androgen Withdrawal Response". Archived from the original on 2005-09-11. Retrieved 2005-08-31.
- ↑ Rabe T, Kowald A, Ortmann J, Rehberger-Schneider S (August 2000). "Inhibition of skin 5 alpha-reductase by oral contraceptive progestins in vitro". Gynecological Endocrinology. 14 (4): 223–30. doi:10.3109/09513590009167685. PMID 11075290.
- ↑ Stárka L, Sulcová J, Broulík P (1976). "Effect of cyproterone acetate on the action and metabolism of testosterone in the mouse kidney". Endokrinologie. 68 (2): 155–63. PMID 1009901.
- ↑ Raudrant D, Rabe T (2003). "Progestogens with antiandrogenic properties". Drugs. 63 (5): 463–92. doi:10.2165/00003495-200363050-00003. PMID 12600226.
- ↑ Tartagni M, Schonauer LM, De Salvia MA, Cicinelli E, De Pergola G, D'Addario V (April 2000). "Comparison of Diane 35 and Diane 35 plus finasteride in the treatment of hirsutism". Fertility and Sterility. 73 (4): 718–23. doi:10.1016/s0015-0282(99)00633-0. PMID 10731531.
- ↑ Sahin Y, Dilber S, Keleştimur F (March 2001). "Comparison of Diane 35 and Diane 35 plus finasteride in the treatment of hirsutism". Fertility and Sterility. 75 (3): 496–500. doi:10.1016/s0015-0282(00)01764-7. PMID 11239530.
- 1 2 Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 133–134. ISBN 978-3-642-73790-9.
- ↑ Herbert DC, Schuppler J, Poggel A, Günzel P, El Etreby MF (1977). "Effect of cyproterone acetate on prolactin secretion in the female Rhesus monkey". Cell Tissue Res. 183 (1): 51–60. doi:10.1007/bf00219991. PMID 411573.
- 1 2 3 4 Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW (January 2000). "Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men". The American Journal of Surgical Pathology. 24 (1): 74–80. doi:10.1097/00000478-200001000-00009. PMID 10632490.
- 1 2 Lawrence, Anne A. (2007). "Transgender Health Concerns". The Health of Sexual Minorities: 473–505. doi:10.1007/978-0-387-31334-4_19.
- 1 2 Paul Peter Rosen (2009). Rosen's Breast Pathology. Lippincott Williams & Wilkins. pp. 31–. ISBN 978-0-7817-7137-5.
- ↑ Lorincz AM, Sukumar S (2006). "Molecular links between obesity and breast cancer". Endocrine-related Cancer. 13 (2): 279–92. doi:10.1677/erc.1.00729. PMID 16728564.
Adipocytes make up the bulk of the human breast, with epithelial cells accounting for only approximately 10% of human breast volume.
- ↑ Howard BA, Gusterson BA (2000). "Human breast development". Journal of Mammary Gland Biology and Neoplasia. 5 (2): 119–37. doi:10.1023/a:1026487120779. PMID 11149569.
In the stroma, there is an increase in the amount of fibrous and fatty tissue, with the adult nonlactating breast consisting of 80% or more of stroma.
- ↑ Sperling MA (10 April 2014). Pediatric Endocrinology. Elsevier Health Sciences. pp. 598–. ISBN 978-1-4557-5973-6.
Estrogen stimulates the nipples to grow, mammary terminal duct branching to progress to the stage at which ductules are formed, and fatty stromal growth to increase until it constitutes about 85% of the mass of the breast. [...] Lobulation appears around menarche, when multiple blind saccular buds form by branching of the terminal ducts. These effects are due to the presence of progesterone. [...] Full alveolar development normally only occurs during pregnancy under the influence of additional progesterone and prolactin.
- ↑ Hagisawa S, Shimura N, Arisaka O (2012). "Effect of excess estrogen on breast and external genitalia development in growth hormone deficiency". Journal of Pediatric and Adolescent Gynecology. 25 (3): e61–3. doi:10.1016/j.jpag.2011.11.005. PMID 22206682.
Estrogen stimulates growth of the nipples, progression of mammary duct branching to the stage at which ductiles are formed, and fatty stromal growth until it constitutes about 85% of the mass of the breast.
- ↑ Wierckx K, Gooren L, T'Sjoen G (May 2014). "Clinical review: Breast development in trans women receiving cross-sex hormones". The Journal of Sexual Medicine. 11 (5): 1240–7. doi:10.1111/jsm.12487. PMID 24618412.
- ↑ Fourcade, R.-O.; McLeod, D. (2015). "Tolerability of Antiandrogens in the Treatment of Prostate Cancer". UroOncology. 4 (1): 5–13. doi:10.1080/1561095042000191655. ISSN 1561-0950.
- 1 2 3 Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- ↑ Donald RA, Espiner EA, Cowles RJ, Fazackerley JE (April 1976). "The effect of cyproterone acetate on the plasma gonadotrophin response to gonadotrophin releasing hormone". Acta Endocrinologica. 81 (4): 680–4. PMID 769466.
- 1 2 Moltz L, Römmler A, Post K, Schwartz U, Hammerstein J (April 1980). "Medium dose cyproterone acetate (CPA): effects on hormone secretion and on spermatogenesis in men". Contraception. 21 (4): 393–413. doi:10.1016/s0010-7824(80)80017-5. PMID 6771095.
- 1 2 3 Rost A, Schmidt-Gollwitzer M, Hantelmann W, Brosig W (1981). "Cyproterone acetate, testosterone, LH, FSH, and prolactin levels in plasma after intramuscular application of cyproterone acetate in patients with prostatic cancer". The Prostate. 2 (3): 315–22. doi:10.1002/pros.2990020310. PMID 6458025.
- ↑ Jeffcoate WJ, Matthews RW, Edwards CR, Field LH, Besser GM (August 1980). "The effect of cyproterone acetate on serum testosterone, LH, FSH, and prolactin in male sexual offenders". Clinical Endocrinology. 13 (2): 189–95. doi:10.1111/j.1365-2265.1980.tb01041.x. PMID 6777092.
- ↑ Grunwald K, Rabe T, Schlereth G, Runnebaum B (November 1994). "[Serum hormones before and during therapy with cyproterone acetate and spironolactone in patients with androgenization]". Geburtshilfe Und Frauenheilkunde (in German). 54 (11): 634–45. doi:10.1055/s-2007-1022355. PMID 8719011.
- ↑ Salva P, Morer F, Ordoñez J, Rodriguez J (1983). "Treatment of idiopathic hirsute women with two combinations of cyproterone acetate". International Journal of Clinical Pharmacology Research. 3 (2): 129–35. PMID 6237068.
- ↑ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- ↑ Wenderoth, U. K.; Jacobi, G. H. (1983). "Gonadotropin-releasing hormone analogues for palliation of carcinoma of the prostate". World Journal of Urology. 1 (1): 40–48. doi:10.1007/BF00326861. ISSN 0724-4983.
- ↑ Anita H. Payne; Matthew P. Hardy (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 426–. ISBN 978-1-59745-453-7.
- ↑ E. Nieschlag; Ursula-F. Habenicht (17 April 2013). Spermatogenesis — Fertilization — Contraception: Molecular, Cellular and Endocrine Events in Male Reproduction. Springer Science & Business Media. pp. 485–. ISBN 978-3-662-02815-5.
- ↑ Koch UJ, Lorenz F, Danehl K, Ericsson R, Hasan SH, Keyserlingk DV, Lübke K, Mehring M, Römmler A, Schwartz U, Hammerstein J (1976). "Continuous oral low-dosage cyproterone acetate for fertility regulation in the male? A trend analysis in 15 volunteers". Contraception. 14 (2): 117–35. doi:10.1016/0010-7824(76)90081-0. PMID 949890.
- ↑ Moltz, L.; Römmler, A.; Schwartz, U.; Hammerstein, J. (1978). "Effects of Cyproterone Acetate (CPA) on Pituitary Gonadotrophin Release and on Androgen Secretion Before and After LH-RH Double Stimulation Tests in Men". International Journal of Andrology. 1 (s2b): 713–719. doi:10.1111/j.1365-2605.1978.tb00518.x. ISSN 0105-6263.
- ↑ Wang C, Yeung KK (1980). "Use of low-dosage oral cyproterone acetate as a male contraceptive". Contraception. 21 (3): 245–72. doi:10.1016/0010-7824(80)90005-0. PMID 6771091.
- ↑ Knuth UA, Hano R, Nieschlag E (1984). "Effect of flutamide or cyproterone acetate on pituitary and testicular hormones in normal men". J. Clin. Endocrinol. Metab. 59 (5): 963–9. doi:10.1210/jcem-59-5-963. PMID 6237116.
- 1 2 Fung, Raymond; Hellstern-Layefsky, Miriam; Lega, Iliana (2017). "Is a lower dose of cyproterone acetate as effective at testosterone suppression in transgender women as higher doses?". International Journal of Transgenderism. 18 (2): 123–128. doi:10.1080/15532739.2017.1290566. ISSN 1553-2739.
- ↑ Ricardo Azziz (8 November 2007). Androgen Excess Disorders in Women. Springer Science & Business Media. pp. 382–. ISBN 978-1-59745-179-6.
- ↑ Girard J, Baumann JB, Bühler U, Zuppinger K, Haas HG, Staub JJ, et al. (1978). "Cyproteroneacetate and ACTH adrenal function". J. Clin. Endocrinol. Metab. 47 (3): 581–6. doi:10.1210/jcem-47-3-581. PMID 233676.
- 1 2 Panesar NS, Herries DG, Stitch SR (1979). "Effects of cyproterone and cyproterone acetate on the adrenal gland in the rat: studies in vivo and in vitro". J. Endocrinol. 80 (2): 229–38. doi:10.1677/joe.0.0800229. PMID 438696.
- ↑ El Etreby MF (1979). "Effect of cyproterone acetate, levonorgestrel and progesterone on adrenal glands and reproductive organs in the beagle bitch". Cell Tissue Res. 200 (2): 229–43. doi:10.1007/bf00236416. PMID 487397.
- ↑ Savage DC, Swift PG (1981). "Effect of cyproterone acetate on adrenocortical function in children with precocious puberty". Arch. Dis. Child. 56 (3): 218–22. doi:10.1136/adc.56.3.218. PMC 1627152. PMID 6260040.
- ↑ Stivel MS, Kauli R, Kaufman H, Laron Z (1982). "Adrenocortical function in children with precocious sexual development during treatment with cyproterone acetate". Clin. Endocrinol. 16 (2): 163–9. doi:10.1111/j.1365-2265.1982.tb03160.x. PMID 6279337.
- ↑ Hague WM, Munro DS, Sawers RS, Duncan SL, Honour JW (1982). "Long-term effects of cyproterone acetate on the pituitary adrenal axis in adult women". Br J Obstet Gynaecol. 89 (12): 981–4. doi:10.1111/j.1471-0528.1982.tb04650.x. PMID 6216913.
- ↑ Mercier L, Miller PA, Simons SS (1986). "Antiglucocorticoid steroids have increased agonist activity in those hepatoma cell lines that are more sensitive to glucocorticoids". J. Steroid Biochem. 25 (1): 11–20. doi:10.1016/0022-4731(86)90275-x. PMID 2875214.
- ↑ Poulin R, Baker D, Poirier D, Labrie F (1991). "Multiple actions of synthetic 'progestins' on the growth of ZR-75-1 human breast cancer cells: an in vitro model for the simultaneous assay of androgen, progestin, estrogen, and glucocorticoid agonistic and antagonistic activities of steroids". Breast Cancer Research and Treatment. 17 (3): 197–210. doi:10.1007/BF01806369. PMID 1645605.
- ↑ Pham-Huu-Trung MT, de Smitter N, Bogyo A, Girard F (1984). "Effects of cyproterone acetate on adrenal steroidogenesis in vitro". Horm. Res. 20 (2): 108–15. doi:10.1159/000179982. PMID 6237971.
- ↑ Lambert A, Mitchell RM, Robertson WR (1985). "On the site of action of the anti-adrenal steroidogenic effect of cyproterone acetate". Biochem. Pharmacol. 34 (12): 2091–5. doi:10.1016/0006-2952(85)90400-9. PMID 2988566.
- ↑ Heinze F, Teller WM, Fehm HL, Joos A (1978). "The effect of cyproterone acetate on adrenal cortical function in children with precocious puberty". Eur. J. Pediatr. 128 (2): 81–8. doi:10.1007/bf00496993. PMID 208851.
- ↑ Bhargava AS, Seeger A, Günzel P (1977). "Isolation and identification of 15-beta-hydroxy cyproterone acetate as a new metabolite of cyproterone acetate in dog, monkey and man". Steroids. 30 (3): 407–18. doi:10.1016/0039-128x(77)90031-9. PMID 413211.
- 1 2 Bhargava AS, Kapp JF, Poggel HA, Heinick J, Nieuweboer B, Günzel P (1981). "Effect of cyproterone acetate and its metabolites on the adrenal function in man, rhesus monkey and rat". Arzneimittelforschung. 31 (6): 1005–9. PMID 6266428.
- ↑ Broulik PD, Starka L (1975). "Corticosteroid-like effect of cyproterone and cyproterone acetate in mice". Experientia. 31 (11): 1364–5. doi:10.1007/bf01945829. PMID 1204803.
- ↑ van Wayjen RG, van den Ende A (1981). "Effect of cyproterone acetate on pituitary-adrenocortical function in man". Acta Endocrinol. 96 (1): 112–22. doi:10.1530/acta.0.0960112. PMID 6257015.
- ↑ Schürmeyer T, Graff J, Senge T, Nieschlag E (1986). "Effect of oestrogen or cyproterone acetate treatment on adrenocortical function in prostate carcinoma patients". Acta Endocrinol. 111 (3): 360–7. doi:10.1530/acta.0.1110360. PMID 2421511.
- ↑ van Wayjen RG, van den Ende A (1995). "Experience in the long-term treatment of patients with hirsutism and/or acne with cyproterone acetate-containing preparations: efficacy, metabolic and endocrine effects". Exp. Clin. Endocrinol. Diabetes. 103 (4): 241–51. doi:10.1055/s-0029-1211357. PMID 7584530.
- ↑ Holdaway IM, Croxson MS, Evans MC, France J, Sheehan A, Wilson T, et al. (1983). "Effect of cyproterone acetate on glucocorticoid secretion in patients treated for hirsutism". Acta Endocrinol. 104 (2): 222–6. doi:10.1530/acta.0.1040222. PMID 6227191.
- ↑ John A. Thomas (12 March 1997). Endocrine Toxicology, Second Edition. CRC Press. pp. 152–. ISBN 978-1-4398-1048-4.
- ↑ Nick Panay (31 August 2015). Managing the Menopause. Cambridge University Press. pp. 126–. ISBN 978-1-107-45182-7.
- ↑ Schröder FH (1998). "Antiandrogens as monotherapy for prostate cancer". European Urology. 34 Suppl 3: 12–7. doi:10.1159/000052291. PMID 9854190.
- 1 2 Gutiérrez M, Menéndez L, Ruiz-Gayo M, Hidalgo A, Baamonde A (June 1997). "Cyproterone acetate displaces opiate binding in mouse brain". European Journal of Pharmacology. 328 (1): 99–102. doi:10.1016/s0014-2999(97)83034-8. PMID 9203575.
- ↑ Gruber CJ, Huber JC (December 2003). "Differential effects of progestins on the brain". Maturitas. 46 Suppl 1: S71–5. doi:10.1016/j.maturitas.2003.09.021. PMID 14670648.
- 1 2 3 Kuhnz W, Kulmann H, Fuhrmeister A (1997). "Investigation into the age-dependence of the pharmacokinetics of cyproterone acetate in healthy male volunteers". Eur. J. Clin. Pharmacol. 53 (1): 75–80. doi:10.1007/s002280050340. PMID 9349934.
- ↑ Hümpel M, Wendt H, Schulze PE, Dogs G, Weiss C, Speck U (May 1977). "Bioavailability and pharmacokinetics of cyproterone acetate after oral administration of 2.0 mg cyproterone acetate in combination with 50 micrograms ethinyloestradiol to 6 young women". Contraception. 15 (5): 579–88. doi:10.1016/0010-7824(77)90108-1. PMID 880829.
- ↑ Hümpel, M.; Wendt, H.; Dogs, G.; Weiβ, Chr.; Rietz, S.; Speck, U. (1977). "Intraindividual comparison of pharmacokinetic parameters of d-norgestrel, lynestrenol and cyproterone acetate in 6 women". Contraception. 16 (2): 199–215. doi:10.1016/0010-7824(77)90087-7. ISSN 0010-7824.
- ↑ Düsterberg B, Hümpel M, Wendt H (1979). "Plasma levels of active ingredients after single and repeated administration of a new oral contraceptive containing 2 mg of cyproterone acetate and 50 micrograms of ethinyl estradiol (DIANE) to five young women". Acta Obstet Gynecol Scand Suppl. 88: 27–31. doi:10.3109/00016347909157226. PMID 294111.
- ↑ Kuhnz W, Staks T, Jütting G (December 1993). "Pharmacokinetics of cyproterone acetate and ethinylestradiol in 15 women who received a combination oral contraceptive during three treatment cycles". Contraception. 48 (6): 557–75. doi:10.1016/0010-7824(93)90118-Q. PMID 8131397.
- ↑ Frölich M, Vader HL, Walma ST, de Rooy HA (September 1980). "Cyproterone acetate in blood of hirsute women during long-term treatment. The absorption and elimination after oral application". J. Steroid Biochem. 13 (9): 1097–100. doi:10.1016/0022-4731(80)90142-9. PMID 7421247.
- ↑ Denmeade SR, Isaacs JT (May 2002). "A history of prostate cancer treatment". Nat. Rev. Cancer. 2 (5): 389–96. doi:10.1038/nrc801. PMC 4124639. PMID 12044015.
- ↑ Schindler AE (2015). "Pharmacology of Progestogens". In Carp HJ. Progestogens in Obstetrics and Gynecology. Springer. pp. 38–. ISBN 978-3-319-14385-9.
- ↑ Fischl FH. (2001). "Pharmacology of Estrogens and Gestagens" (PDF). In Krause & Pachemegg. Menopause andropause (PDF). Gablitz: Krause und Pachernegg. pp. 33–50. ISBN 3-901299-34-3.
- ↑ New Zealand Medicines; Medical Devices Safety Authority (2005-12-09). "Data Sheet: Diane 35 ED". Archived from the original on 2007-01-08.
- ↑ Medicines and Healthcare products Regulatory Authority (2006-04-11). "Cyproterone Acetate" (PDF).
- ↑ Giorgi EP, Shirley IM, Grant JK, Stewart JC (March 1973). "Androgen dynamics in vitro in the human prostate gland. Effect of cyproterone and cyproterone acetate". The Biochemical Journal. 132 (3): 465–74. doi:10.1042/bj1320465. PMC 1177610. PMID 4125095.
- 1 2 John V. Knaus; John H. Isaacs (6 December 2012). Office Gynecology: Advanced Management Concepts. Springer Science & Business Media. pp. 150–. ISBN 978-1-4612-4340-3.
- 1 2 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1182–1183. ISBN 978-0-8155-1856-3.
- ↑ Duang Buddhasukh, Roland Maier, Aranya Manosroi, Jiradej Manosroi, Pattana Sripalakit, Rolf Werner (29 April 2002). "EP1359154A1 Further syntheses of cyproterone acetate". Google Patents.
- 1 2 https://patents.google.com/patent/DE1189991B
- 1 2 https://patents.google.com/patent/US3234093A
- ↑ U.S. Patent 3,234,093
- ↑ Forsberg JG, Jacobsohn D, Norgren A (1968). "Modifications of reproductive organs in male rats influenced prenatally or pre- and postnatally by an "antiandrogenic" steroid (Cyproterone)". Zeitschrift Für Anatomie Und Entwicklungsgeschichte. Springer. 127 (2): 175–86. doi:10.1007/bf00521983. PMID 5692718.
- ↑ Thibaut F, De La Barra F, Gordon H, Cosyns P, Bradford JM (June 2010). "The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias". World J. Biol. Psychiatry. 11 (4): 604–55. doi:10.3109/15622971003671628. PMID 20459370.
- ↑ Tobias JS, Hochhauser D (3 October 2014). Cancer and its Management. Wiley. pp. 379–. ISBN 978-1-118-46871-5.
- ↑ T. Mann; C. Lutwak-Mann (6 December 2012). Male Reproductive Function and Semen: Themes and Trends in Physiology, Biochemistry and Investigative Andrology. Springer Science & Business Media. pp. 352–. ISBN 978-1-4471-1300-3.
- ↑ Anthony W. Norman; Gerald Litwack (28 June 2014). Hormones. Elsevier Science. pp. 508–. ISBN 978-1-4832-5810-2.
- ↑ W.I.P. Mainwaring (6 December 2012). The Mechanism of Action of Androgens. Springer Science & Business Media. pp. 53–. ISBN 978-3-642-88429-0.
- ↑ Neumann F, Wiechert R (March 1988). "[The antiandrogen cyproterone acetate. Its history from discovery to marketing]". Pharm Unserer Zeit (in German). 17 (2): 33–50. PMID 2967500.
- 1 2 3 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 339–. ISBN 978-1-4757-2085-3.
- 1 2 3 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 89–. ISBN 978-94-011-4439-1.
- ↑ Christoph Zink (1 January 1988). Dictionary of Obstetrics and Gynecology. Walter de Gruyter. pp. 61–. ISBN 978-3-11-085727-6.
- ↑ F. William Danby (27 January 2015). Acne: Causes and Practical Management. John Wiley & Sons. pp. 142–. ISBN 978-1-118-23277-4.
- 1 2 3 4 5 6 http://www.micromedexsolutions.com/micromedex2/%5Bpermanent+dead+link%5D
- 1 2 3 4 5 6 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- ↑ Jack H. Mydlo; Ciril J. Godec (11 July 2003). Prostate Cancer: Science and Clinical Practice. Academic Press. pp. 437–. ISBN 978-0-08-049789-1.
- 1 2 V. Unzeitig; Rick H.W. van Lunsen (15 February 2000). Contraceptive Choices and Realities: Proceedings of the 5th Congress of the European Society of Contraception. CRC Press. pp. 73–. ISBN 978-1-85070-067-8.
- 1 2 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 44, 437. ISBN 978-92-832-1291-1.
- ↑ John David Gordon (2007). Obstetrics, Gynecology & Infertility: Handbook for Clinicians. Scrub Hill Press, Inc. pp. 228–. ISBN 978-0-9645467-7-6.
- ↑ Cox RL, Crawford ED (December 1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/S0022-5347(01)66670-9. PMID 7500443.
- 1 2 Goldenberg SL, Bruchovsky N, Rennie PS, Coppin CM (December 1988). "The combination of cyproterone acetate and low dose diethylstilbestrol in the treatment of advanced prostatic carcinoma". J. Urol. 140 (6): 1460–5. doi:10.1016/S0022-5347(17)42073-8. PMID 2973529.
- ↑ Matzkin H, Braf Z (January 1991). "Endocrine treatment of benign prostatic hypertrophy: current concepts". Urology. 37 (1): 1–16. PMID 1702565.
- ↑ Tenaglia R, Nicolai M, Di Federico G, Iantorno R, Zezza A, Lombardi G (1993). "[Androgen deprivation in benign prostatic hypertrophy]". J Urol (Paris) (in French). 99 (6): 296–8. PMID 7516371.
- ↑ Di Silverio F, D'Eramo G, Flammia GP, Buscarini M, Frascaro E, Mariani M, Sciarra A (1993). "[Pharmacological combinations in the treatment of benign prostatic hypertrophy]". J Urol (Paris) (in French). 99 (6): 316–20. PMID 7516378.
- ↑ Nieschlag E (2010). "Clinical trials in male hormonal contraception". Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
- 1 2 http://adisinsight.springer.com/drugs/800015250
- ↑ Geier DA, Kern JK, King PG, Sykes LK, Geier MR (2012). "An evaluation of the role and treatment of elevated male hormones in autism spectrum disorders". Acta Neurobiol Exp (Wars). 72 (1): 1–17. PMID 22508080.
- ↑ Bolea-Alamanac BM, Davies SJ, Christmas DM, Baxter H, Cullum S, Nutt DJ (2011). "Cyproterone to treat aggressivity in dementia: a clinical case and systematic review". J. Psychopharmacol. (Oxford). 25 (1): 141–5. doi:10.1177/0269881109353460. PMID 19942637.
- ↑ Albert DJ, Walsh ML, Jonik RH (1993). "Aggression in humans: what is its biological foundation?". Neurosci Biobehav Rev. 17 (4): 405–25. doi:10.1016/S0149-7634(05)80117-4. PMID 8309650.
- ↑ Thibaut F, Kuhn JM, Colonna L (August 1991). "A possible antiaggressive effect of cyproterone acetate". Br J Psychiatry. 159: 298–9. doi:10.1192/bjp.159.2.298b. PMID 1837749.
- ↑ Judith L. Rapoport (1 January 1989). Obsessive-compulsive Disorder in Children and Adolescents. American Psychiatric Pub. pp. 229–231. ISBN 978-0-88048-282-0.
- ↑ Kellner M (2010). "Drug treatment of obsessive-compulsive disorder". Dialogues in Clinical Neuroscience. 12 (2): 187–97. PMC 3181958. PMID 20623923.
- ↑ López Ibor JJ, Cercós CL, Masiá CC (2004). Images of Spanish Psychiatry. Editorial Glosa, S.L. pp. 376–. ISBN 978-84-7429-200-8.
- ↑ Sicuteri F (1988). "Antiandrogenic medication of cluster headache". Int J Clin Pharmacol Res. 8 (1): 21–4. PMID 3366500.
Further reading
- Goldenberg SL, Bruchovsky N (1991). "Use of cyproterone acetate in prostate cancer". Urol. Clin. North Am. 18 (1): 111–22. PMID 1825143.
- Neumann F, Kalmus J (1991). "Cyproterone acetate in the treatment of sexual disorders: pharmacological base and clinical experience". Exp. Clin. Endocrinol. 98 (2): 71–80. doi:10.1055/s-0029-1211103. PMID 1838080.
- Schröder FH (1993). "Cyproterone acetate--mechanism of action and clinical effectiveness in prostate cancer treatment". Cancer. 72 (12 Suppl): 3810–5. doi:10.1002/1097-0142(19931215)72:12+<3810::aid-cncr2820721710>3.0.co;2-o. PMID 8252496.
- Barradell LB, Faulds D (1994). "Cyproterone. A review of its pharmacology and therapeutic efficacy in prostate cancer". Drugs Aging. 5 (1): 59–80. doi:10.2165/00002512-199405010-00006. PMID 7919640.
- Neumann F (1994). "The antiandrogen cyproterone acetate: discovery, chemistry, basic pharmacology, clinical use and tool in basic research" (PDF). Exp. Clin. Endocrinol. 102 (1): 1–32. doi:10.1055/s-0029-1211261. PMID 8005205.
- Laron Z, Kauli R (2000). "Experience with cyproterone acetate in the treatment of precocious puberty". J. Pediatr. Endocrinol. Metab. 13 Suppl 1: 805–10. doi:10.1515/jpem.2000.13.s1.805. PMID 10969925.
- Van der Spuy ZM, le Roux PA (2003). "Cyproterone acetate for hirsutism". Cochrane Database Syst Rev (4): CD001125. doi:10.1002/14651858.CD001125. PMID 14583927.
- Torri V, Floriani I (2005). "Cyproterone acetate in the therapy of prostate carcinoma". Arch Ital Urol Androl. 77 (3): 157–63. PMID 16372511.
External links
- [The History of Cyproterone Acetate: From the "Pill for Men" to the "Skin Care Product and Contraceptive"] - Arznei-Telegramm (in German) [Google Translate]