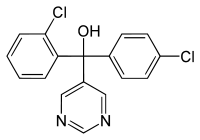
Fenarimol
 | |
| Names | |
|---|---|
| IUPAC name
(R/S)-2,4′-Dichloro-α-(pyrimidin-5-yl)benzhydryl alcohol | |
| Other names
α-(2-Chlorophenyl)-α-(4-chlorophenyl)-5-pyrimidinemethanol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.056.432 |
PubChem CID |
|
| |
| |
| Properties | |
| C17H12Cl2N2O | |
| Molar mass | 331.20 g·mol−1 |
| Appearance | Colorless powder with aromatic odor |
| Melting point | 117 to 119 °C (243 to 246 °F; 390 to 392 K)[1] |
| Boiling point | 240 °C (464 °F; 513 K) (decomposition)[1] |
| 13.7 mg·L−1 at 25 °C | |
| Solubility in other solvents | Soluble in acetone, xylene and methanol[1] |
| Vapor pressure | 65 μ Pa (25 °C)[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
>2000 mg·kg−1 (oral, Ratte)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fenarimol, sold under the tradenames Bloc, Rimidin and Rubigan, is a fungicide which acts against rusts, blackspot and mildew fungi. It is used on ornamental plants, trees, lawns, tomatoes, peppers, eggplants, cucumbers and melons. It is mainly used to control powdery mildew. It works by inhibiting the fungus's biosynthesis of important steroid molecules.[2]
Description
Fenarimol was developed by Eli Lilly & Company around 1971.[3]
- Health Risk
Fenarimol can lead to increased growth of MCF7 breast cancer cells.[4] It has been found to be an endocrine disruptor, acting as a xenoestrogen and antiandrogen.[5]
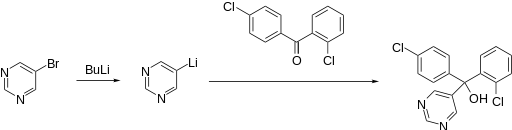
Synthesis
Fenarimol is made by the reaction of a diarylketone with an organolithium derived by halogen-metal exchange.[2]
References
- 1 2 3 4 5 EU-Data.
- 1 2 Clayden J, Greeves N, Warren S (2005). Organic chemistry (Reprinted (with corrections) ed.). Oxford [u.a.]: Oxford Univ. Press. p. 216. ISBN 978-0-19-850346-0.
- ↑ GB 1218623 "Substituted-5-pyrimidine compounds "
- ↑ Vinggaard, Anne Marie; Breinholt, Vibeke; Larsen, John Christian (1 December 1999). "Screening of selected pesticides for oestrogen receptor activation in vitro". Food Additives and Contaminants. 16 (12): 533–542. doi:10.1080/026520399283678. PMID 10789375.
- ↑ Raun Andersen, Helle; Vinggaard, Anne Marie; Høj Rasmussen, Thomas; Gjermandsen, Irene Marianne; Cecilie Bonefeld-Jørgensen, Eva (2002). "Effects of Currently Used Pesticides in Assays for Estrogenicity, Androgenicity, and Aromatase Activity in Vitro". Toxicology and Applied Pharmacology. 179 (1): 1–12. doi:10.1006/taap.2001.9347. ISSN 0041-008X. PMID 11884232.
External links
- Fenarimol in the Pesticide Properties DataBase (PPDB)
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.