Esterified estrogens
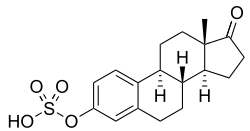 Estrone sulfate, the primary active component in esterified estrogens (constitutes about 75 to 85% of total content). | |
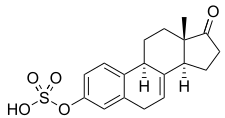 Equilin sulfate, the second most major active component in esterified estrogens (constitutes about 6 to 16% of total content). | |
| Combination of | |
|---|---|
| Sodium estrone sulfate | Estrogen |
| Sodium equilin sulfate | Estrogen |
| Clinical data | |
| Trade names | Estratab, Menest, others |
| Routes of administration | By mouth[1] |
| Drug class | Estrogen |
| ATC code |
|
| Identifiers | |
| PubChem SID | |
| DrugBank | |
| UNII | |
Esterified estrogens (EEs), sold under the brand names Estratab and Menest among others, is an estrogen medication which is used hormone therapy for menopausal symptoms and low sex hormone levels in women, to treat breast cancer in both women and men, and to treat prostate cancer in men.[2][3][4][5][6] It is formulated alone or in combination with methyltestosterone.[2][3] It is taken by mouth.[1]
Side effects of EEs include nausea, breast tension, edema, and breakthrough bleeding among others.[7] It is an estrogen, or an agonist of the estrogen receptors, the biological target of estrogens like estradiol.[4][2][3] EEs are a prodrug mainly of estradiol and to a lesser extent of equilin.[4]
EEs were introduced for medical use by 1970.[8] They are available in only a few countries, such as Chile and the United States.[2] They have also been marketed in Argentina and Switzerland in the past.[2]
Medical uses
EEs are used in hormone therapy for menopausal symptoms, female hypogonadism, ovariectomy, and primary ovarian failure and in the treatment of breast cancer and prostate cancer.[3][9]
Available forms
EEs are available in the form of 0.3 mg, 0.625 mg, 1.25 mg, and 2.5 mg oral tablets.[10] Estratest is a combination formulation of 1.25 mg EEs with 2.5 mg methyltestosterone.[11]
Side effects
Pharmacology
EEs consist primarily of sodium estrone sulfate and sodium equilin sulfate, and are very similar to conjugated estrogens (CEEs; brand name Premarin).[4][6][12][13] However, EEs and CEEs differ in the sources of their contents and in the percentages of their constituents; CEEs consist of approximately 53% sodium estrone sulfate and 25% sodium equilin sulfate, while EEs contain about 75 to 85% sodium estrone sulfate and 6 to 11% sodium equilin sulfate.[4][2][12][14][9] EEs have been found to produce similar serum levels of estrone and estradiol relative to CEEs, although with higher levels of estrone and lower levels of equilin.[4][15] One study found that the risk of venous thrombosis may be less with EEs relative to CEEs.[14][6]
| Estrogen | Type | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Bioidentical | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | Bioidentical | ND | ND | ND | 0.3 | 0.3 | ND | ND | ND | ND | ND |
| Estriol | Bioidentical | 0.3 | 0.3 | 0.1 | 0.3 | ND | 0.2 | ND | ND | ND | 0.67 |
| Estrone sulfate | Bioidentical | ND | 0.9 | 0.9 | 0.9 | 0.9 | 0.5 | 0.9 | 0.7 | 1.5 | 0.56–1.7 |
| Conjugated estrogens | Natural | 1.2 | 1.5 | 2.0 | 1.1 | 1.0 | 1.5 | 3.0 | 1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | Natural | ND | ND | ND | ND | ND | 6.0 | 7.5 | 6.0 | 7.5 | ND |
| Ethinylestradiol | Synthetic | 120 | 150 | 40 | 120 | 100 | 400 | 500 | 600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | Synthetic | ND | ND | ND | 3.4 | ND | ND | 25.6 | 24.5 | 19.5 | 5.7–7.5 |
| Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these hepatic proteins. Ratio = Ratio of liver protein effects to hot flashes relief and gonadotropin suppression. ND = No data. Type: Bioidentical = Identical to those found in humans. Natural = Naturally occurring but not identical to those found in humans (e.g., estrogens of other species). Synthetic = Man-made, does not naturally occur in animals or in the environment. Miscellaneous: Direct link to table. Sources: [4][16][17][18][19][20] | |||||||||||
| Compound | RBA to SHBG (%) | Bound to SHBG (%) | Bound to albumin (%) | Total bound (%) | MCR (L/day/m2) |
|---|---|---|---|---|---|
| 17β-Estradiol | 50 | 37 | 61 | 98 | 580 |
| Estrone | 12 | 16 | 80 | 96 | 1050 |
| Estriol | 0.3 | 1 | 91 | 92 | 1110 |
| Estrone sulfate | 0 | 0 | 99 | 99 | 80 |
| 17β-Dihydroequilin | 30 | ? | ? | ? | 1250 |
| Equilin | 8 | 26 | 13 | ? | 2640 |
| 17β-Dihydroequilin sulfate | 0 | ? | ? | ? | 375 |
| Equilin sulfate | 0 | ? | ? | ? | 175 |
| Δ8-Estrone | ? | ? | ? | ? | 1710 |
| Notes: RBA to SHBG (%) is compared to 100% for testosterone. Source:[4] Miscellaneous: Direct link to table. | |||||
Chemistry
EEs contain synthetic, plant-derived estrogens and are manufactured from soybeans and yams.[5][6]
History
EEs were introduced for medical use by 1970.[8]
Society and culture
Generic names
Estrogens, esterified is the generic name of the drug and its USP.[21] It is also known as esterified estrogens.[3]
Brand names
EEs are marketed under a variety of brand names including Amnestrogen, Estragyn, Estratab, Evex, Femibel, Femogen, Menest, Neo Estrone Tab, and Oestro-Feminal alone, and, in combination with methyltestosterone, under the brand names Covaryx, Delitan, Eemt, Essian, Estratest, Feminova-T, Menogen, and Syntest.[2][5][3]
Availability
EEs are or have been marketed in Argentina, Chile, Switzerland, and the United States.[2] Both EEs and the combination of EEs and methyltestosterone are listed as being marketed only in Chile and the United States as of present.[2]
See also
References
- 1 2 Katherine Sherif (14 May 2013). Hormone Therapy: A Clinical Handbook. Springer Science & Business Media. pp. 120–. ISBN 978-1-4614-6268-2.
- 1 2 3 4 5 6 7 8 9 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2097. ISBN 978-0-85369-840-1.
- 1 2 3 4 5 6 https://www.drugbank.ca/drugs/DB09381
- 1 2 3 4 5 6 7 8 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 3 Carl P. Weiner, MD; Kate Rope (2 April 2013). The Complete Guide to Medications During Pregnancy and Breastfeeding: Everything You Need to Know to Make the Best Choices for You and Your Baby. St. Martin's Press. pp. 179–. ISBN 978-0-312-67646-9.
- 1 2 3 4 Smith, N. L.; Heckbert, S. R.; Lemaitre, R. N.; Reiner, A. P.; Lumley, T.; Rosendaal, F. R.; Psaty, B. M. (2006). "Conjugated Equine Estrogen, Esterified Estrogen, Prothrombotic Variants, and the Risk of Venous Thrombosis in Postmenopausal Women". Arteriosclerosis, Thrombosis, and Vascular Biology. 26 (12): 2807–2812. doi:10.1161/01.ATV.0000245792.62517.3b. ISSN 1079-5642.
- ↑ Wittlinger, H. (1980). "Clinical Effects of Estrogens": 67–71. doi:10.1007/978-3-642-67568-3_10.
- 1 2 Northwest Medicine. Northwest Medical Pub. Association. 1970.
- 1 2 Manuchair Ebadi (31 October 2007). Desk Reference of Clinical Pharmacology, Second Edition. CRC Press. pp. 249–. ISBN 978-1-4200-4744-8.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 30 March 2018.
- ↑ John E. Morley; Lucretia van den Berg (5 November 1999). Endocrinology of Aging. Springer Science & Business Media. pp. 172–. ISBN 978-1-59259-715-4.
- 1 2 Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 752–. ISBN 978-1-4511-4847-3.
- ↑ Tara Parker-Pope (9 January 2007). The Hormone Decision: Untangle the Controversy, Understand Your Options, Make Your Own Choices. Rodale. pp. 157–. ISBN 978-1-59486-927-3.
- 1 2 Smith, Nicholas L. (2004). "Esterified Estrogens and Conjugated Equine Estrogens and the Risk of Venous Thrombosis". JAMA. 292 (13): 1581. doi:10.1001/jama.292.13.1581. ISSN 0098-7484.
- ↑ Lemaitre, Rozenn N.; Weiss, Noel S.; Smith, Nicholas L.; Psaty, Bruce M.; Lumley, Thomas; Larson, Eric B.; Heckbert, Susan R. (2006). "Esterified Estrogen and Conjugated Equine Estrogen and the Risk of Incident Myocardial Infarction and Stroke". Archives of Internal Medicine. 166 (4): 399. doi:10.1001/archinte.166.4.399. ISSN 0003-9926. PMID 16505258.
- ↑ Alfred S. Wolf; H.P.G. Schneider (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 78–. ISBN 978-3-642-75101-1.
- ↑ Manfred Kaufmann; Serban-Dan Costa; Anton Scharl (27 November 2013). Die Gynäkologie. Springer-Verlag. pp. 105–. ISBN 978-3-662-11496-4.
- ↑ Mashchak CA, Lobo RA, Dozono-Takano R, Eggena P, Nakamura RM, Brenner PF, Mishell DR (November 1982). "Comparison of pharmacodynamic properties of various estrogen formulations". Am. J. Obstet. Gynecol. 144 (5): 511–8. doi:10.1016/0002-9378(82)90218-6. PMID 6291391.
- ↑ Helgason S (1982). "Estrogen replacement therapy after the menopause. Estrogenicity and metabolic effects". Acta Obstet Gynecol Scand Suppl. 107: 1–29. doi:10.3109/00016348209155333. PMID 6282033.
- ↑ Lobo RA, Nguyen HN, Eggena P, Brenner PF (February 1988). "Biologic effects of equilin sulfate in postmenopausal women". Fertil. Steril. 49 (2): 234–8. doi:10.1016/S0015-0282(16)59708-8. PMID 3338581.
- ↑ https://chem.nlm.nih.gov/chemidplus/sid/d042724000