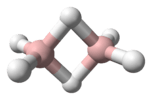
Propene
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Propene[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.693 | ||
| KEGG | |||
PubChem CID |
|||
| RTECS number | UC6740000 | ||
| UN number | 1077 In Liquefied petroleum gas: 1075 | ||
| |||
| |||
| Properties | |||
| C3H6 | |||
| Molar mass | 42.08 g·mol−1 | ||
| Appearance | Colorless gas | ||
| Density | 1.81 kg/m3, gas (1.013 bar, 15 °C) 613.9 kg/m3, liquid | ||
| Melting point | −185.2 °C (−301.4 °F; 88.0 K) | ||
| Boiling point | −47.6 °C (−53.7 °F; 225.6 K) | ||
| 0.61 g/m3 | |||
| -31.5·10−6 cm3/mol | |||
| Viscosity | 8.34 µPa·s at 16.7 °C | ||
| Structure | |||
| 0.366 D (gas) | |||
| Hazards | |||
| Safety data sheet | External MSDS | ||
EU classification (DSD) (outdated) |
|||
| R-phrases (outdated) | 12 | ||
| S-phrases (outdated) | 9-16-33 | ||
| NFPA 704 | |||
| Flash point | −108 °C (−162 °F; 165 K) | ||
| Related compounds | |||
Related alkenes; related groups |
Ethylene, Isomers of Butylene; Allyl, Propenyl | ||
Related compounds |
Propane, Propyne Propadiene, 1-Propanol 2-Propanol | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Propene, also known as propylene or methyl ethylene, is an unsaturated organic compound having the chemical formula . It has one double bond, and is the second simplest member of the alkene class of hydrocarbons. It is a colorless gas with a faint petroleum-like odor[2]
Production
Propene is a byproduct of oil refining and natural gas processing. During oil refining, ethylene, propene, and other compounds are produced as a result of cracking larger hydrocarbons. A major source of propene is naphtha cracking intended to produce ethylene, but it also results from refinery cracking producing other products.[3] Propene can be separated by fractional distillation from hydrocarbon mixtures obtained from cracking and other refining processes; refinery-grade propene is about 50 to 70%.[3]
A shift to lighter steam cracker feedstocks with relatively lower propene yields and reduced motor gasoline demand in certain areas has reduced propene supply.
On-purpose production methods are significant.[4] On-purpose production technologies include:
The Phillips Triolefin and the Olefin Conversion Processes interconverts propylene with ethylene and 2-butenes. Rhenium and molybdenum catalysts are used. The conversion of ethylene and 2-butene to propylene is industrially practiced.[5][4] Propene yields of about 90 wt% are achieved.
Propane dehydrogenation (PDH) converts propane into propene and by-product hydrogen. The propene from propane yield is about 85 m%. Reaction by-products (mainly hydrogen) are usually used as fuel for the propane dehydrogenation reaction. As a result, propene tends to be the only product, unless local demand exists for hydrogen. This route is popular in regions, such as the Middle East, where there is an abundance of propane from oil/gas operations.[6] In this region, the propane output is expected to be capable of supplying not only domestic needs, but also the demand from China, where many PDH projects are scheduled to go on stream. However, as natural gas offerings in the United States are significantly increasing due to the rising exploitation of shale gas, propane prices are decreasing. Chemical companies are already planning to establish PDH plants in the USA to take advantage of the low price raw material, obtained from shale gas. Numerous plants dedicated to propane dehydrogenation are currently under construction around the world. There are already five licensed technologies.[7] The propane dehydrogenation process may be accomplished through different commercial technologies. The main differences between each of them concerns the catalyst employed, design of the reactor and strategies to achieve higher conversion rates.[8]
Methanol-to-Olefins/Methanol-to-Propene converts synthesis gas (syngas) to methanol, and then converts the methanol to ethylene and/or propene. The process produces water as by-product. Synthesis gas is produced from the reformation of natural gas or by the steam-induced reformation of petroleum products such as naphtha, or by gasification of coal.
High severity fluid catalytic cracking (FCC) uses traditional FCC technology under severe conditions (higher catalyst-to-oil ratios, higher steam injection rates, higher temperatures, etc.) in order to maximize the amount of propene and other light products. A high severity FCC unit is usually fed with gas oils (paraffins) and residues, and produces about 20–25 m% propene on feedstock together with greater volumes of motor gasoline and distillate byproducts.
Several companies have explored biomanufacturing using engineered enzymes.[9] The starting materials for the fermentation could be either sugars or petrochemicals.
Propene production has remained static at around 35 million tonnes (Europe and North America only) from 2000 to 2008, but it has been increasing in East Asia, most notably Singapore and China.[10] Total world production of propene is currently about half that of ethylene.
Uses
Propene is the second most important starting product in the petrochemical industry after ethylene. It is the raw material for a wide variety of products. Manufacturers of the plastic polypropylene account for nearly two thirds of all demand.[11] Polypropylene end uses include films, fibers, containers, packaging, and caps and closures. Propene is also used for the production of important chemicals such as propylene oxide, acrylonitrile, cumene, butyraldehyde, and acrylic acid. In the year 2013 about 85 million tonnes of propene were processed worldwide.[11]
Propene and benzene are converted to acetone and phenol via the cumene process.

Propene is also used to produce isopropanol (propan-2-ol), acrylonitrile, propylene oxide, and epichlorohydrin.[12] The industrial production of acrylic acid involves the catalytic partial oxidation of propene.[13] Propene is also an intermediate in the one-step propane selective oxidation to acrylic acid.[14][15][16][17] In industry and workshops, propene is used as an alternative fuel to acetylene in Oxy-fuel welding and cutting, brazing and heating of metal for the purpose of bending. It has become a standard in BernzOmatic products and others in MAPP substitutes,[18] now that true MAPP gas is no longer available.
Reactions
Propene resembles other alkenes in that it undergoes addition reactions relatively easily at room temperature. The relative weakness of its double bond explains its tendency to react with substances that can achieve this transformation. Alkene reactions include: 1) polymerization, 2) oxidation, 3) halogenation and hydrohalogenation, 4) alkylation, 5) hydration, 6) oligomerization, and 7) hydroformylation.
Combustion
Propene undergoes combustion reactions in a similar fashion to other alkenes. In the presence of sufficient or excess oxygen, propene burns to form water and carbon dioxide.
- 2 C3H6 + 9 O2 → 6 CO2 + 6 H2O
When insufficient oxygen is present for complete combustion, incomplete combustion occurs allowing carbon monoxide and/or soot (carbon) to be formed as well.
- C3H6 + 2 O2 → 3 H2O + 2 C + CO
Environmental safety
Propene is a product of combustion from forest fires, cigarette smoke, and motor vehicle and aircraft exhaust. It is an impurity in some heating gases. Observed concentrations have been in the range of 0.1-4.8 parts per billion (ppb) in rural air, 4-10.5 ppb in urban air, and 7-260 ppb in industrial air samples.[3]
In the United States and some European countries a threshold limit value of 500 parts per million (ppm) was established for occupational (8-hour time-weighted average) exposure. It is considered a volatile organic compound (VOC) and emissions are regulated by many governments, but it is not listed by the U.S. Environmental Protection Agency (EPA) as a hazardous air pollutant under the Clean Air Act. With a relatively short half-life, it is not expected to bioaccumulate.[3]
Propene has low acute toxicity from inhalation. Inhalation of the gas can cause anesthetic effects and at very high concentrations, unconsciousness. However, the asphyxiation limit for humans is about 10 times higher (23%) than the lower flammability level.[3]
Storage and handling
Since propene is volatile and flammable, precautions must be taken to avoid fire hazards in the handling of the gas. If propene is loaded to any equipment capable of causing ignition, such equipment should be shut down while loading, unloading, connecting or disconnecting. Propene is usually stored as liquid under pressure, although it is also possible to store it safely as gas at ambient temperature in approved containers.[19]
Pharmacology
Propene acts as a central nervous system depressant via allosteric agonism of the GABAA receptor. Excessive exposure may result in sedation and amnesia, progressing to coma and death in a mechanism equivalent to benzodiazepine overdose. Intentional inhalation may also result in death via asphyxiation (sudden inhalant death).
Occurrence in nature
On September 30, 2013, NASA announced that the Cassini orbiter spacecraft, part of the Cassini-Huygens mission, had discovered small amounts of naturally occurring propene in the atmosphere of Titan using spectroscopy.[20][21]
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 31. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ https://pubchem.ncbi.nlm.nih.gov/compound/Propene#section=Top
- 1 2 3 4 5 "Product Safety Assessment(PSA): Propylene". Dow Chemical Co.
- 1 2 "Propylene Production via Metathesis, Technology Economics Program". by Intratec,
ISBN 978-0-615-61145-7, Q2 2012. templatestyles stripmarker in
|publisher=at position 14 (help) - ↑ Lionel Delaude, Alfred F. Noels (2005). "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Weinheim: Wiley-VCH. doi:10.1002/0471238961.metanoel.a01.
- ↑ Ashford’s Dictionary of Industrial Chemicals, Third edition, 2011, ISBN 978-0-9522674-3-0, pages 7766-9
- ↑ Giovanni Maggini (2012-06-28). "Technology Economics: Propylene via Propane Dehydrogenation". Slideshare.net. Retrieved 2013-11-12.
- ↑ Giovanni Maggini (2013-04-17). "Technology Economics: Propylene via Propane Dehydrogenation, Part 3". Slideshare.net. Retrieved 2013-11-12.
- ↑ de Guzman, Doris (October 12, 2012). "Global Bioenergies in bio-propylene". Green Chemicals Blog.
- ↑ Organic Chemistry 6th edition, McMurry,J., Brooks/Cole Publishing, Pacific Grove USA (2005)
- 1 2 "Market Study: Propylene (2nd edition), Ceresana, December 2014". ceresana.com. Retrieved 2015-02-03.
- ↑ Budavari, Susan, ed. (1996). "8034. Propylene". The Merck Index, Twelfth Edition. New Jersey: Merck & Co. pp. 1348–1349
- ↑ J.G.L., Fierro (Ed.) (2006). Metal Oxides, Chemistry and Applications. CRC Press. p. 414–455.
- ↑ "The reaction network in propane oxidation over phase-pure MoVTeNb M1 oxide catalysts". Journal of Catalysis. 311: 369–385. March 2014. doi:10.1016/j.jcat.2013.12.008.
- ↑ "Multifunctionality of Crystalline MoV(TeNb) M1 Oxide Catalysts in Selective Oxidation of Propane and Benzyl Alcohol". ACS Catalysis. 3 (6): 1103–1113. 7 June 2013. doi:10.1021/cs400010q.
- ↑ "Surface chemistry of phase-pure M1 MoVTeNb oxide during operation in selective oxidation of propane to acrylic acid". Journal of Catalysis. 285 (1): 48–60. January 2012. doi:10.1016/j.jcat.2011.09.012.
- ↑ Kinetic studies of propane oxidation on Mo and V based mixed oxide catalysts. pp. 3–24, 93.
- ↑ For example, "MAPP-Pro"
- ↑ Encyclopedia of Chemical Technology, Fourth edition, 1996, ISBN 0471-52689-4 (v.20), page 261
- ↑ "Spacecraft finds propylene on Saturn moon, Titan". UPI.com. 2013-09-30. Retrieved 2013-11-12.
- ↑ "Cassini finds ingredient of household plastic on Saturn moon". Spacedaily.com. Retrieved 2013-11-12.