SAGE-217
 | |
| Clinical data | |
|---|---|
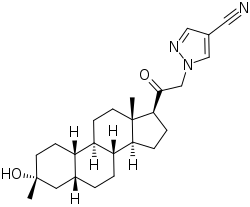
| Synonyms | 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1'-yl)-19-nor-5β-pregnan-20-one; 3β-Methyl-21-(4-cyano-1H-pyrazol-1'-yl)-19-norpregnanolone; 3α-Hydroxy-3β-methyl-5β-dihydro-21-(4-cyano-1H-pyrazol-1'-yl)-19-norprogesterone |
| Routes of administration | By mouth |
| Drug class | Neurosteroid; GABAA receptor positive allosteric modulator |
| Identifiers | |
| |
| Chemical and physical data | |
| Formula | C25H35N3O2 |
| Molar mass | 409.273 g/mol |
| 3D model (JSmol) | |
| |
| |
SAGE-217 is an investigational medication which is under development by SAGE Therapeutics for the treatment of major depressive disorder, postpartum depression, essential tremor, Parkinson's disease, insomnia, and seizures.[1][2] It is a synthetic, orally active, inhibitory pregnane neurosteroid, and acts as a positive allosteric modulator of the GABAA receptor.[1][2][3] The drug was developed as an improvement of allopregnanolone (brexanolone) with high oral bioavailability and a biological half-life suitable for once-daily administration.[2] As of February 2018, SAGE-217 is in phase II clinical trials for major depressive disorder, postpartum depression, essential tremor, and Parkinson's disease and is in phase I clinical studies for insomnia and seizures.[1] It is also in the preclinical stage of development for dyskinesias.[1]
See also
References
- 1 2 3 4 "SAGE 217". AdisInsight. Retrieved 2018-02-10.
- 1 2 3 Blanco MJ, La D, Coughlin Q, Newman CA, Griffin AM, Harrison BL, Salituro FG (2018). "Breakthroughs in neuroactive steroid drug discovery". Bioorg. Med. Chem. Lett. 28 (2): 61–70. doi:10.1016/j.bmcl.2017.11.043. PMID 29223589.
- ↑ Martinez Botella G, Salituro FG, Harrison BL, Beresis RT, Bai Z, Blanco MJ, Belfort GM, Dai J, Loya CM, Ackley MA, Althaus AL, Grossman SJ, Hoffmann E, Doherty JJ, Robichaud AJ (2017). "Neuroactive Steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1H-pyrazol-1'-yl)-19-nor-5β-pregnan-20-one (SAGE-217): A Clinical Next Generation Neuroactive Steroid Positive Allosteric Modulator of the (γ-Aminobutyric Acid)A Receptor". J. Med. Chem. 60 (18): 7810–7819. doi:10.1021/acs.jmedchem.7b00846. PMID 28753313.