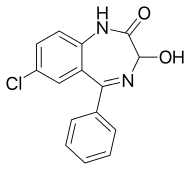
Oxazepam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Serax, generics |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95.5% |
| Metabolism | Hepatic |
| Elimination half-life | 5–15 h[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.009.161 |
| Chemical and physical data | |
| Formula | C15H11ClN2O2 |
| Molar mass | 286.71 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 205 to 206 °C (401 to 403 °F) |
| |
| |
| (verify) | |
Oxazepam is a short-to-intermediate-acting benzodiazepine.[3][4] Oxazepam is used for the treatment of anxiety [5][6] and insomnia and in the control of symptoms of alcohol withdrawal syndrome.
It is a metabolite of diazepam, prazepam, and temazepam,[7] and has moderate amnesic, anxiolytic, anticonvulsant, hypnotic, sedative, and skeletal muscle relaxant properties compared to other benzodiazepines.[8] Oxazepam was initially patented and marketed in 1965 under the brand name Serax.[9]
Medical uses
It is an intermediate-acting benzodiazepine with a slow onset of action,[10] so it is usually prescribed to individuals who have trouble staying asleep, rather than falling asleep. It is commonly prescribed for anxiety disorders with associated tension, irritability, and agitation. It is also prescribed for drug and alcohol withdrawal, and for anxiety associated with depression. Physicians may use oxazepam outside its approved indications to treat social phobia, post-traumatic stress disorder, insomnia, premenstrual syndrome, and other conditions.[11]

Use
Oxazepam, along with diazepam, nitrazepam, and temazepam, were the four benzodiazepines listed on the pharmaceutical benefits scheme and represented 82% of the benzodiazepine prescriptions in Australia in 1990-1991.[12] In Finland it is customary to use one 15 milligram tablet for a singular dose, 1 to 3 times a day, making the dose 1x15mg=15mg, 2x15mg=30mg or 3x15mg=45g. The tablet can be halved if there is the need to take 7.5 mg perhaps because the patient is an elder person (a senior citizen), or a child. It is usually prescribed on an as-needed basis. If this is the case, then it is optimal to take no oxazepam at all, because long-term use can have adverse reactions and consequences on the user.
If the medicine is prescribed to be taken on a must-take basis, then the advice of the doctor needs to be followed closely, but usually it is 1x15mg in the morning, 1x15mg during the day and 1x15mg during the evening.
Side effects
The side effects of oxazepam are similar to those of other benzodiazepines, and may include dizziness, drowsiness, headache, memory impairment, paradoxical excitement, and anterograde amnesia, but does not affect transient global amnesia. Side effects due to rapid decrease in dose or abrupt withdrawal from oxazepam may include abdominal and muscle cramps, convulsions, depression, inability to fall asleep or stay asleep, sweating, tremors, or vomiting.[13]
Tolerance, dependence and withdrawal
Oxazepam, as with other benzodiazepine drugs, can cause tolerance, physical dependence, addiction, and benzodiazepine withdrawal syndrome. Withdrawal from oxazepam or other benzodiazepines often leads to withdrawal symptoms which are similar to those seen during alcohol and barbiturate withdrawal. The higher the dose and the longer the drug is taken, the greater the risk of experiencing unpleasant withdrawal symptoms. Withdrawal symptoms can occur, though, at standard dosages and also after short-term use. Benzodiazepine treatment should be discontinued as soon as possible by a slow and gradual dose reduction regimen.[14]
When the dose is titrated for a withdrawal (done for health reasons), tapered off there is first 1x7.5mg (half a tablet) during the day when the morning and night are usually kept with the usual dose 1x15mg, and then use the dose for 3 days and then one half is taken away from the morning dose, and then wait for three days with that dose, then one half from the evening, and then there is no oxazepam during the day (zero milligrams), and then zero in the morning, then zero altogether. The tapering of the dose is done under health care supervision. It takes about 18 days to get rid of the medicine (drug), but if done right there should be no problems. The dose adjustment must be done under a clinical doctor's advice and supervision, and the nurses need to know how the person is coping with the dose adjustment.
Contraindications
Oxazepam is contraindicated in myasthenia gravis, chronic obstructive pulmonary disease, and limited pulmonary reserve, as well as severe hepatic disease.
Special precautions
Benzodiazepines require special precautions if used in the elderly, during pregnancy, in children, alcohol- or drug-dependent individuals, and individuals with comorbid psychiatric disorders.[15] Benzodiazepines including oxazepam are lipophilic drugs and rapidly penetrate membranes, so rapidly crosses over into the placenta with significant uptake of the drug. Use of benzodiazepines in late pregnancy, especially high doses, may result in floppy infant syndrome.[16]
Pregnancy
Oxazepam when taken during late in pregnancy, the third trimester, causes a definite risk to the neonate including a severe benzodiazepine withdrawal syndrome including hypotonia, and reluctance to suck, to apnoeic spells, cyanosis, and impaired metabolic responses to cold stress. Floppy infant syndrome and sedation in the newborn may also occur. Symptoms of floppy infant syndrome and the neonatal benzodiazepine withdrawal syndrome have been reported to persist from hours to months after birth.[17]
Interactions
As oxazepam is an active metabolite of diazepam, an overlap in possible interactions is likely with other drugs or food, with exception of the pharmacokinetic CYP450 interactions (e.g. with cimetidine). Precautions and following the prescription are required when taking oxazepam (or other benzodiazepines) in combinations with antidepressant medication (SSRIs such as fluoxetine, sertraline, and paroxetine, or multiple reuptake inhibitors such as bupropion, duloxetine, or venlafaxine), potent painkillers (opioids, e.g. morphine, oxycodone or methadone). Concurrent use of these medicines (as well as other benzodiazepines) can interact in a way that is difficult to predict. Drinking alcohol when taking oxazepam is not recommended. Concomitant use of oxazepam and alcohol can lead to increased sedation, severe problems with coordination (ataxia), decreased muscle tone, and in severe cases or in predisposed patients, even to life-threatening intoxications with respiratory depression, coma, and collapse.
Overdose
Oxazepam is generally less toxic in overdose than other benzodiazepines.[18] Important factors which affect the severity of a benzodiazepine overdose include the dose ingested, the age of the patient, and health status prior to overdose. Benzodiazepine overdoses can be much more dangerous if a coingestion of other CNS depressants such as opiates or alcohol has occurred. Symptoms of an oxazepam overdose include:[19][20][21]
- Respiratory depression
- Excessive somnolence
- Altered consciousness
- Central nervous system depression
- Occasionally cardiovascular and pulmonary toxicity
- Rarely, deep coma
Pharmacology
Oxazepam is an intermediate-acting benzodiazepine of the 3-hydroxy family; it acts on benzodiazepine receptors, resulting in increased effect of GABA to the GABAA receptor which results in inhibitory effects on the central nervous system.[22][23] The half-life of oxazepam is four to 15 hours.[24] It has been shown to suppress cortisol levels.[25] Oxazepam is the most slowly absorbed and has the slowest onset of action of all the common benzodiazepines according to one British study.[26]
Oxazepam is an active metabolite formed during the breakdown of diazepam, nordazepam, and certain similar drugs. It may be safer than many other benzodiazepines in patients with impaired liver function because it does not require hepatic oxidation, but rather, it is simply metabolized by glucuronidation, so oxazepam is less likely to accumulate and cause adverse reactions in the elderly or people with liver disease. Oxazepam is similar to lorazepam in this respect. (1) Preferential storage of oxazepam occurs in some organs, including the heart of the neonate. Absorption by any administered route and the risk of accumulation is significantly increased in the neonate, and withdrawal of oxazepam during pregnancy and breast feeding is recommended, as oxazepam is excreted in breast milk.[27]
Chemistry
Oxazepam exists as a racemic mixture.[28] Early attempts to isolate enantiomers were unsuccessful; the corresponding acetate has been isolated as a single enantiomer. Given the different rates of epimerization that occur at different pH levels, it was determined that there would be no therapeutic benefit to the administration of a single enantiomer over the racemic mixture.[29]
Society and culture
Misuse
Oxazepam has the potential for misuse, defined as taking the drug to achieve a high, or continuing to take the drug in the long term against medical advice.[30] Benzodiazepines, including diazepam, oxazepam, nitrazepam, and flunitrazepam, accounted for the largest volume of forged drug prescriptions in Sweden from 1982 to 1986. During this time, a total of 52% of drug forgeries were for benzodiazepines, suggesting they were a major prescription drug class of abuse.[31]
However, due to its slow rate of absorption and its slow onset of action,[26] oxazepam has a relatively low potential for abuse compared to some other benzodiazepines, such as temazepam, flunitrazepam, or triazolam, which have a high potential for abuse similar to barbiturates.[32]
A benzodiazepine high can remind of (mimic) a cannabis high, but usually benzodiazepines do not produce hallucinations, like some other substances can. Also, benzodiazepines are a bit like dry versions of alcohol, and it is often the case that if the medicine is used as a drug against the advice of health care and police authorities, the dose is very high as measured in milligrams, not just one tablet but several. People can and do die of benzodiazepines. Oxazepam particularly is a drug that can be used as a mildly euphoric sedative to help sleep or to be more social and outgoing, but used for a long time it makes a person joyless and tired, and kills his or her feelings. It is a good idea to keep long pauses when using oxazepam or another benzodiazepine, a drug in this class. The ideal is not to use benzodiazepines at all. If the benzo (like oxazepam) is never started that all, that is optimal. If it is started, it should be used with as low a dose as possible as little a time as possible, either for a short period of time or several short periods of time with long times of abstinence from the drug in between. Benzodiazepines like oxazepam are not candy, they are used for severely anxious people's neurotic symptoms.
it is optimal to take no oxazepam at all, because long-term use can have adverse reactions and consequences on the user. Oxazepam is not an antipsychotic. Neuroleptic antipsychotic medications are antipsychotic and the main course of treatment for psychosis like schizophrenia or bipolar psychosis or manic or depressive psychosis.
Oxazepam and other benzodiazepine derivatives are often abused in combination with alcohol, and that can result in death (suicide, accidents, violence, motor vehicle accidents, respiratory depression). That kind of use is considered polydrug abuse.
Legal status
Oxazepam is a Schedule IV drug under the Convention on Psychotropic Substances.[33]
Brand names
It is marketed under many brand names worldwide, including: Alepam, Alepan, Anoxa, Anxiolit, Comedormir, durazepam, Murelax, Nozepam, Oksazepam, Opamox, Ox-Pam, Oxa-CT, Oxabenz, Oxamin, Oxapam, Oxapax, Oxascand, Oxaze, Oxazepam, Oxazépam, Oxazin, Oxepam, Praxiten, Purata, Selars, Serenal, Serepax, Seresta, Séresta, Serpax, Sobril, Tazepam, Vaben, and Youfei.[34]
It is also marketed in combination with scopolamine as Novalona and in combination with alanine as Pausafrent T.[34]
References
- ↑ Greenblatt DJ (1981). "Clinical pharmacokinetics of oxazepam and lorazepam". Clin Pharmacokinet. 6 (2): 89–105. doi:10.2165/00003088-198106020-00001. PMID 6111408.
- ↑ CID 4616 from PubChem
- ↑ "Benzodiazepine Names". non-benzodiazepines.org.uk. Archived from the original on 2008-12-08. Retrieved 2008-12-29.
- ↑ "FASS". Läkemedelsindustriföreningens Service AB. Archived from the original on 2011-10-01. Retrieved 2011-02-03.
- ↑ Janecek, James; Vestre, Norris D.; Schiele, Burtrum C.; Zimmermann, Robert (1966). "Oxazepam in the treatment of anxiety states: A controlled study". Journal of Psychiatric Research. 4 (3): 199–206. doi:10.1016/0022-3956(66)90007-0. ISSN 0022-3956.
- ↑ Sarris, J.; Scholey, A.; Schweitzer, I.; Bousman, C.; Laporte, E.; Ng, C.; Murray, G.; Stough, C. (2012). "The acute effects of kava and oxazepam on anxiety, mood, neurocognition; and genetic correlates: a randomized, placebo-controlled, double-blind study". Human Psychopharmacology. 27 (3): 262–269. doi:10.1002/hup.2216. ISSN 1099-1077. PMID 22311378.
- ↑ "Oxazepam (IARC Summary & Evaluation, Volume 66, 1996)". IARC. Archived from the original on 2008-09-07. Retrieved 2009-03-12.
- ↑ Mandrioli R, Mercolini L, Raggi MA (October 2008). "Benzodiazepine metabolism: an analytical perspective". Curr. Drug Metab. 9 (8): 827–44. doi:10.2174/138920008786049258. PMID 18855614. Archived from the original on 2009-03-17.
- ↑ Shorter, Edward (2005). "B". A Historical Dictionary of Psychiatry. Oxford University Press. ISBN 9780190292010.
- ↑ Galanter, Marc; Kleber, Herbert D. (1 July 2008). The American Psychiatric Publishing Textbook of Substance Abuse Treatment (4th ed.). United States of America: American Psychiatric Publishing Inc. p. 216. ISBN 978-1-58562-276-4.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2011-07-15. Retrieved 2009-04-22.
- ↑ Mant A; Whicker SD; McManus P; Birkett DJ; Edmonds D; Dumbrell D. (December 1993). "Benzodiazepine utilisation in Australia: report from a new pharmacoepidemiological database". Aust J Public Health. 17 (4): 345–9. doi:10.1111/j.1753-6405.1993.tb00167.x. PMID 7911332.
- ↑ "Oxazepam Uses, Side Effects & Warnings - Drugs.com". drugs.com. Archived from the original on 2009-06-04.
- ↑ MacKinnon GL; Parker WA. (1982). "Benzodiazepine withdrawal syndrome: a literature review and evaluation". The American Journal of Drug and Alcohol Abuse. 9 (1): 19–33. doi:10.3109/00952998209002608. PMID 6133446.
- ↑ Authier, N.; Balayssac, D.; Sautereau, M.; Zangarelli, A.; Courty, P.; Somogyi, AA.; Vennat, B.; Llorca, PM.; Eschalier, A. (November 2009). "Benzodiazepine dependence: focus on withdrawal syndrome". Ann Pharm Fr. 67 (6): 408–13. doi:10.1016/j.pharma.2009.07.001. PMID 19900604.
- ↑ Kanto JH. (May 1982). "Use of benzodiazepines during pregnancy, labour and lactation, with particular reference to pharmacokinetic considerations". Drugs. 23 (5): 354–80. doi:10.2165/00003495-198223050-00002. PMID 6124415.
- ↑ McElhatton PR. (Nov–Dec 1994). "The effects of benzodiazepine use during pregnancy and lactation". Reprod Toxicol. 8 (6): 461–75. doi:10.1016/0890-6238(94)90029-9. PMID 7881198.
- ↑ Buckley NA, Dawson AH, Whyte IM, O'Connell DL (28 January 1995). "Relative toxicity of benzodiazepines in overdose". BMJ. 310 (6974): 219–21. doi:10.1136/bmj.310.6974.219. PMC 2548618. PMID 7866122. Archived from the original on 13 January 2010.
- ↑ Gaudreault P, Guay J, Thivierge RL, Verdy I (1991). "Benzodiazepine poisoning. Clinical and pharmacological considerations and treatment". Drug Saf. 6 (4): 247–65. doi:10.2165/00002018-199106040-00003. PMID 1888441.
- ↑ Perry HE, Shannon MW (June 1996). "Diagnosis and management of opioid- and benzodiazepine-induced comatose overdose in children". Current Opinion in Pediatrics. 8 (3): 243–7. doi:10.1097/00008480-199606000-00010. PMID 8814402.
- ↑ Busto U, Kaplan HL, Sellers EM (February 1980). "Benzodiazepine-associated emergencies in Toronto". Am J Psychiatry. 137 (2): 224–7. doi:10.1176/ajp.137.2.224. PMID 6101526.
- ↑ Skerritt JH; Johnston GA. (May 6, 1983). "Enhancement of GABA binding by benzodiazepines and related anxiolytics". Eur J Pharmacol. 89 (3–4): 193–8. doi:10.1016/0014-2999(83)90494-6. PMID 6135616.
- ↑ Oelschläger H. (July 4, 1989). "[Chemical and pharmacologic aspects of benzodiazepines]". Schweiz Rundsch Med Prax. 78 (27–28): 766–72. PMID 2570451.
- ↑ Professor heather Ashton (April 2007). "Benzodiazepine equivalency table". Archived from the original on September 28, 2007. Retrieved September 23, 2007.
- ↑ Christensen P; Lolk A; Gram LF; Kragh-Sørensen P. (1992). "Benzodiazepine-induced sedation and cortisol suppression. A placebo-controlled comparison of oxazepam and nitrazepam in healthy male volunteers". Psychopharmacology. 106 (4): 511–6. doi:10.1007/BF02244823. PMID 1349754.
- 1 2 Serfaty M, Masterton G (1993). "Fatal poisonings attributed to benzodiazepines in Britain during the 1980s". Br J Psychiatry. 163 (3): 386–93. doi:10.1192/bjp.163.3.386. PMID 8104653.
- ↑ Olive G; Dreux C. (January 1977). "Pharmacologic bases of use of benzodiazepines in peréinatal medicine". Arch Fr Pediatr. 34 (1): 74–89. PMID 851373.
- ↑ Aso, Yukio; et al. (1988). "The Kinetics of the Racemization of Oxazepam in Aqueous Solution". Chemical and Pharmaceutical Bulletin. 36 (5): 1834–1840. doi:10.1248/cpb.36.1834. Retrieved September 9, 2016.
- ↑ Crossley, Roger J. (1995). Chirality and Biological Activity of Drugs. CRC Press. ISBN 0849391407.
- ↑ Griffiths RR, Johnson MW (2005). "Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds". J Clin Psychiatry. 66 Suppl 9: 31–41. PMID 16336040.
- ↑ Bergman U; Dahl-Puustinen ML. (1989). "Use of prescription forgeries in a drug abuse surveillance network". Eur J Clin Pharmacol. 36 (6): 621–3. doi:10.1007/BF00637747. PMID 2776820.
- ↑ Griffiths RR, Wolf B (August 1990). "Relative abuse liability of different benzodiazepines in drug abusers". J Clin Psychopharmacol. 10 (4): 237–43. doi:10.1097/00004714-199008000-00002. PMID 1981067.
- ↑ "Archived copy" (PDF). Archived (PDF) from the original on 2005-12-05. Retrieved 2005-11-19.
- 1 2 "Oxazepam - International Brand Names". Drugs.com. Archived from the original on 5 January 2017. Retrieved 4 January 2017.