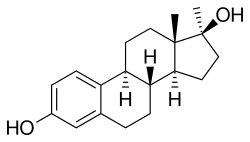
Methylestradiol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ginecosid, Ginecoside, Mediol, Renodiol |
| Synonyms | NSC-52245; 17α-Methylestradiol; 17α-ME; 17α-Methylestra-1,3,5(10)-triene-3,17β-diol |
| Routes of administration | By mouth[1] |
| Drug class | Estrogen |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H26O2 |
| Molar mass | 286.415 g/mol |
| 3D model (JSmol) | |
| |
| |
Methylestradiol, sold under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, is an estrogen medication which is used in the treatment of menopausal symptoms.[2][3][4] It is formulated in combination with normethandrone, a progestin and androgen/anabolic steroid medication.[3][4] Methylestradiol is taken by mouth.[1]
Side effects of methylestradiol include nausea, breast tension, edema, and breakthrough bleeding among others.[5] It is an estrogen, or an agonist of the estrogen receptors, the biological target of estrogens like estradiol.[6]
Methylestradiol is or has been marketed in Brazil, Venezuela, and Indonesia.[3] In addition to its use as a medication, methylestradiol has been studied for use as a radiopharmaceutical for the estrogen receptor.[7]
Medical uses
Methylestradiol is used in combination with the progestin and androgen/anabolic steroid normethandrone (methylestrenolone) in the treatment of menopausal symptoms.[3][4]
Available forms
Methylestradiol is marketed in combination with normethandrone in the form of oral tablets containing 0.3 mg methylestradiol and 5 mg normethandrone.[8][9]
Side effects
Side effects of methylestradiol include nausea, breast tension, edema, and breakthrough bleeding.[5]
Pharmacology
Pharmacodynamics
Methylestradiol is an estrogen, or an agonist of the estrogen receptor.[6] It shows somewhat lower affinity for the estrogen receptor than estradiol or ethinylestradiol.[6]
Methylestradiol is an active metabolite of the androgens/anabolic steroids methyltestosterone (17α-methyltestosterone), metandienone (17α-methyl-δ1-testosterone), and normethandrone (17α-methyl-19-nortestosterone), and is responsible for their estrogenic side effects, such as gynecomastia and fluid retention.[10][11][12]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | ||
|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7 | <0.1 | ||
| Ethinylestradiol | 15–25 | 1–3 | 112 | 1–3 | <1 | ? | ? | ||
| Methylestradiol | 3–10, 15–25 | 1–3 | 67 | 1–3 | <1 | ? | ? | ||
| Methyltestosterone | 3 | 45, 100–125 | ? | 1–5 | ? | 5 | ? | ||
| Normethandrone | 100 | 146 | <0.1 | 1.5 | 0.6 | ? | ? | ||
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. | |||||||||
Pharmacokinetics
Due to the presence of its C17α methyl group, methylestradiol cannot be deactivated by oxidation of the C17β hydroxyl group, resulting in improved metabolic stability and potency relative to estradiol.[10] This is analogous to the case of ethinylestradiol and its C17α ethynyl group.[10]
Chemistry
Methylestradiol, or 17α-methylestradiol (17α-ME), also known as 17α-methylestra-1,3,5(10)-triene-3,17β-diol, is a synthetic estrane steroid and a derivative of estradiol.[2][3] It is specifically the derivative of estradiol with a methyl group at the C17α positions.[2][3] Closely related steroids include ethinylestradiol (17α-ethynylestradiol) and ethylestradiol (17α-ethylestradiol).[2] The C3 cyclopentyl ether of methylestradiol has been studied and shows greater oral potency than methylestradiol in animals, similarly to quinestrol (ethinylestradiol 3-cyclopentyl ether) and quinestradol (estriol 3-cyclopentyl ether).[16]
Society and culture
Generic names
Methylestradiol has not been assigned an INN or other formal name designations.[2][3] Its generic name in English and German is methylestradiol, in French is méthylestradiol, and in Spanish is metilestadiol.[3] It is also known as 17α-methylestradiol.[3]
Brand names
Methylestradiol is or has been marketed under the brand names Ginecosid, Ginecoside, Mediol, and Renodiol, all in combination with normethandrone.[3][2]
Availability
Methylestradiol is or has been marketed in Brazil, Venezuela, and Indonesia.[3]
References
- 1 2 HEGEMANN O (May 1959). "[Oral hormonal treatment with methylestrene-olone & methylestradiol as early pregnancy tests]". Medizinische (in German). 4 (21): 1032–3. PMID 13673847.
- 1 2 3 4 5 6 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 898–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 8 9 10 11 Drugs.com. "Methylestradiol". Retrieved 2 January 2016.
- 1 2 3 IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 389–. ISBN 978-92-832-1291-1.
- 1 2 Wittlinger, H. (1980). "Clinical Effects of Estrogens": 67–71. doi:10.1007/978-3-642-67568-3_10.
- 1 2 3 4 Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Res. 38 (11 Pt 2): 4186–98. PMID 359134.
- ↑ Feenstra A, Vaalburg W, Nolten GM, Reiffers S, Talma AG, Wiegman T, van der Molen HD, Woldring MG (1983). "Estrogen receptor binding radiopharmaceuticals: II. Tissue distribution of 17 alpha-methylestradiol in normal and tumor-bearing rats". J. Nucl. Med. 24 (6): 522–8. PMID 6406650.
- ↑ Unlisted Drugs. Pharmaceutical Section, Special Libraries Association. 1982.
Batynid. C. Each dragee contains: normethandrone, 5 mg.; and methylestradiol, 0.3 mg. E. (Formerly) Gynaekosid. M. Boehringer Biochemia, Florence. A. Estrogenic; Rx of secondary amenorrhea. R. Notiz Med Farm 32;295, Nov-Dec 81.
- ↑ Akingba JB, Ayodeji EA (February 1966). "Amenorrhea as a leading symptom of choriocarcinoma". J Obstet Gynaecol Br Commonw. 73 (1): 153–5. doi:10.1111/j.1471-0528.1966.tb05137.x. PMID 5948541.
- 1 2 3 Detlef Thieme; Peter Hemmersbach (18 December 2009). Doping in Sports. Springer Science & Business Media. pp. 470–. ISBN 978-3-540-79088-4.
- ↑ William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 533–. ISBN 978-0-9828280-1-4.
- ↑ Friedl KE (1990). "Reappraisal of the health risks associated with the use of high doses of oral and injectable androgenic steroids". NIDA Res. Monogr. 102: 142–77. PMID 1964199.
- ↑ Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. (1979). "Receptor Binding as a Tool in the Development of New Bioactive Steroids": 169–214. doi:10.1016/B978-0-12-060308-4.50010-X.
- ↑ Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ↑ Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". J. Steroid Biochem. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- ↑ Falconi, G., Rossi, G. L., & Ercoli, A. (1970). Quinestrol and other cyclopentyl ethers of estrogenic steroids: different rates of storage in body fat. https://www.popline.org/node/474468