Gestonorone caproate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Depostat, Primostat |
| Synonyms | Gestronol hexanoate; Norhydroxyprogesterone caproate; SH-582; SH-80582; NSC-84054; 17α-Hydroxy-19-norpregn-4-ene-3,20-dione hexanoate; 17α-Hydroxy-19-norprogesterone hexanoate |
| Routes of administration | Intramuscular injection[1][2][3] |
| Drug class | Progestin; Progestogen; Progestogen ester; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
Oral: Low[4] IM: High[5] |
| Metabolism | Reduction (at the C5, C3, and C20 positions)[6] |
| Metabolites |
• 19-Norpregnanetriol[6] • 19-Norpregnanediol-20-one[6] |
| Elimination half-life | IM: 7.5 ± 3.1 days[5] |
| Duration of action | IM: ≥21 days[5] |
| Excretion |
Urine: 28%[5] Feces: 72%[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.013.646 |
| Chemical and physical data | |
| Formula | C26H38O4 |
| Molar mass | 414.578 g/mol |
| 3D model (JSmol) | |
| |
| |
Gestonorone caproate, also known as gestronol hexanoate or norhydroxyprogesterone caproate and sold under the brand names Depostat and Primostat, is a progestin medication which is used in the treatment of enlarged prostate, endometrial cancer, and breast cancer.[5][3][7][1][8] It is not effective by mouth and must be given by injection into muscle, typically once a week.[4]
Side effects of gestonorone caproate include worsened glucose tolerance, decreased libido in men, and injection site reactions.[5] Gestonorone caproate is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[9][10] It has no other important hormonal activity.[5][11][12][13]
Gestonorone caproate was discovered in 1960 and was introduced for medical use by 1973.[14][15] It has been used widely throughout Europe, including in the United Kingdom, and has also been marketed in certain other countries such as Japan, China, and Mexico.[1][16][17][18] However, it has since mostly been discontinued, and it remains available today only in a handful of countries, including the Czech Republic, Japan, Mexico, and Russia.[18][19]
Medical uses
Gestonorone caproate is used in the palliative treatment of benign prostatic hypertrophy and advanced endometrial cancer and breast cancer.[5][3][20] It is used at a dose of 100 to 200 mg once a week by intramuscular injection.[5]
Side effects
Side effects of gestonorone caproate have been reported to include worsened glucose tolerance, decreased libido in men, and local injection site reactions such as irritation.[5]
Pharmacology
Pharmacodynamics
Gestonorone caproate is a potent, long-acting, and pure progestogen,[9][10][21] possessing no androgenic, anabolic, antiandrogenic, estrogenic, antiestrogenic, glucocorticoid, mineralocorticoid, or teratogenic effects.[5][11][12][13][21][22] When all are given by subcutaneous injection, it is approximately 20 to 25 times more potent than progesterone and hydroxyprogesterone caproate in animal bioassays.[5][13][21][23] In humans, 100 or 200 mg intramuscular gestonorone caproate has been said to be equivalent to 1,000 mg intramuscular hydroxyprogesterone caproate.[24][25]
Like other potent progestins, gestonorone caproate possesses potent antigonadotropic activity and is capable of markedly suppressing the gonadal production and circulating levels of sex hormones such as testosterone and estradiol.[13][26][27] A clinical study found that 400 mg/week intramuscular gestonorone caproate suppressed testosterone levels by 75% in men, while orchiectomy as a comparator reduced testosterone levels by 91%.[28][29] Levels of luteinizing hormone, conversely, remained unchanged.[28] In accordance with its lack of glucocorticoid activity, gestonorone caproate has no anticorticotropic effects, and does not influence the secretion of adrenocorticotropic hormone.[5]
17α-Hydroxyprogesterone has weak progestogenic activity, but C17α esterification results in higher progestogenic activity.[6] Of a variety of different esters, the caproate (hexanoate) ester was found to have the strongest progestogenic activity, and this formed the basis for the development of gestonorone caproate, as well as other caproate progestogen esters such as hydroxyprogesterone caproate.[6]
Gestonorone caproate has been found to decrease the weights of the prostate gland and seminal vesicles by 40 to 70% in adult male rats.[5] It has been shown in canines to mediate these effects both via its antigonadotropic effects and by direct actions in these tissues.[5] Gestonorone caproate decreases the uptake of testosterone into the prostate gland.[5] It has also been found to have direct antiproliferative effects on human ovarian cancer cells in vitro.[5]
Gestonorone caproate has been reported to act to some extent as a 5α-reductase inhibitor, similarly to progesterone.[30][31]
| Progestogen | Type | Class | TFD (14 days) | MDT (week) | OID (month) | POIC-D (2–3 months) | CIC-D (month) | Duration |
|---|---|---|---|---|---|---|---|---|
| Algestone acetophenide | Synthetic | Pregnane | ND | ND | ND | NA | 75–150 mg | ND |
| Gestonorone caproate | Synthetic | Norpregnane | ND | ND | ND | NA | NA | ND |
| Hydroxyprogesterone caproate | Synthetic | Pregnane | 250–500 mg | 25 mg | 250–500 mg | NA | 250–500 mg | 250 mg ≈ 10 days |
| Medroxyprogesterone acetate | Synthetic | Pregnane | 50–100 mg | ND | ND | 150 mg | 25 mg | 50 mg ≈ 14 days |
| Megestrol acetate | Synthetic | Pregnane | ND | ND | ND | NA | 25 mg | ND |
| Norethisterone enanthate | Synthetic | Estrane | ND | ND | ND | 200 mg | 50 mg | ND |
| Progesterone (oil soln.) | Bioidentical | Pregnane | 200 mg | ND | ND | NA | NA | 25 mg ≈ 2–3 days |
| Progesterone (cryst. susp.) | Bioidentical | Pregnane | 50–100 mg | ND | ND | NA | NA | 50 mg ≈ 14 days |
| Notes: All by intramuscular injection. Abbreviations: TFD = Endometrial transformation dose. MDT = Menstrual delay test dose (Greenblatt). OID = Ovulation-inhibiting dose (antigonadotropic effect; without an estrogen). POIC-D = Progestogen-only injectable contraceptive dose(s). CIC-D = Combined injectable contraceptive dose(s). Miscellaneous: Direct link to table. Sources:[32][33][34][35][36] | ||||||||
Pharmacokinetics
Like the closely related progestins hydroxyprogesterone caproate and 19-norprogesterone, gestonorone caproate shows poor activity orally and must be administered parenterally; specifically, via intramuscular injection.[4] Gestonorone caproate is administered by intramuscular injection, and acts as a long-lasting depot by this route.[5][37][38][39] After an intramuscular injection, gestonorone caproate is completely released from the local depot and is highly bioavailable.[5] At high doses, the duration of action of gestonorone caproate by intramuscular injection has been found to be at least 21 days.[5] Clinical studies have found gestonorone caproate to be satisfactorily effective as a progestogen when injected once a month, whereas it was poorly effective as an injectable contraceptive when it was injected once every two months.[40][41]
Following a single intramuscular injection of 200 mg radiolabeled gestonorone caproate in 1 mL of solution in men with prostate cancer, maximal levels of gestonorone caproate occurred after 3 ± 1 days and were 420 ± 160 ng/mL.[5] The elimination half-life of gestonorone caproate and its metabolites was 7.5 ± 3.1 days.[5] Approximately 5% of the radioactive steroid content in the blood was unchanged gestonorone caproate.[5] No free gestonorone was observed in circulation or in urine.[5] Gestonorone caproate and its metabolites were eliminated 72% in feces and 28% in urine.[5] Approximately 48 ± 18% of the injected dose had been eliminated after 14 days and approximately 85 ± 12% of the injected dose had been excreted after 30 days.[5]
The metabolism of unesterified gestonorone (17α-hydroxy-19-norprogesterone) is analogous to that of 17α-hydroxyprogesterone, with the corresponding 19-norpregnane metabolites produced.[6] Gestonorone caproate has been found to undergo 5α-reduction similarly to progesterone, 17α-hydroxyprogesterone, and gestonorone, and at a similar rate as these steroids.[6] Conversely however, due to its caproate ester, 5β-reduction of gestonorone caproate is decreased relative to these steroids.[6] As progesterone is metabolized mainly into 5β-pregnanes, decreased 5β-reduction of gestonorone caproate may be involved in its greater potency compared to progesterone.[6] The major metabolites of gestonorone caproate have been reported to be isomers of 19-norpregnanetriol and 19-norpregnanediol-20-one.[6][22] These metabolites indicate that gestonorone caproate is metabolized mainly by reduction at the C3, C5, and C20 positions.[6] Following an intramuscular injection of 300 mg gestonorone caproate, only a slight increase in urinary pregnanetriol excretion has been observed.[6] Cleavage of the caproate ester of gestonorone caproate is minimal, which indicates that it is not a prodrug of the unesterified steroid.[6]
Chemistry
Gestonorone caproate, also known as norhydroxyprogesterone caproate, 17α-hydroxy-19-norprogesterone 17α-hexanoate, or 17α-hydroxy-19-norpregn-4-ene-3,20-dione 17α-hexanoate, is a synthetic norpregnane steroid and a derivative of progesterone.[42][16] It is specifically a combined derivative of 17α-hydroxyprogesterone and 19-norprogesterone, or of gestronol (17α-hydroxy-19-norprogesterone), with a hexanoate (caproate) ester at the C17α position.[42][16] Analogues and derivatives of gestonorone caproate include algestone acetophenide (dihydroxyprogesterone acetophenide), demegestone, nomegestrol acetate, norgestomet, and segesterone acetate, as well as 18-methylsegesterone acetate and the caproate esters chlormadinone caproate, hydroxyprogesterone caproate, medroxyprogesterone caproate, megestrol caproate, and methenmadinone caproate.[42][16]
Synthesis
Chemical syntheses of gestonorone caproate have been published.[5][7][43]
History
Gestonorone caproate was first described in 1960.[14] It was developed by Schering and has been marketed since at least 1973.[15]
Society and culture
Generic names
Gestonorone caproate is the generic name of the drug and its INN, USAN, and JAN, while gestronol hexanoate is its BANM.[42][16] It has also been referred to as norhydroxyprogesterone caproate, and is also known by its former developmental code names SH-582 and SH-80582.[42][16][17]
Brand names
Gestonorone caproate has been marketed exclusively under the brand names Depostat and Primostat.[42][16][17][18][19]
Availability
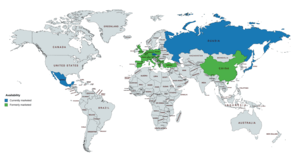
Gestonorone caproate has been available widely in Europe, including in the United Kingdom, and has also been marketed in Japan, China, Mexico, and certain other countries.[1][16][17][18] However, it has been discontinued in most countries and its availability is more limited today; it appears to remain marketed only in the Czech Republic, Japan, Mexico, and Russia.[18][19][44] It has not been marketed in the United States, Canada, and many other countries.[16][17][18][19]
Research
SH-834 was a combination of 90 mg estradiol valerate and 300 mg gestonorone caproate for weekly intramuscular injection that was developed by Schering in the 1970s.[45][46] It was investigated clinically as a treatment for breast cancer and was found to be effective, but does not seem to have been marketed.[45][47][48]
Gestonorone caproate was studied by Schering for use as a progestogen-only injectable contraceptive at a dose of 2.5 to 200 mg once every one or two months but was never marketed.[41][49][50][51][52][53][54][55] There is very little clinical experience of gestonorone caproate for this indication.[41]
Gestonorone caproate has been studied in the treatment of ovarian cancer (in combination with cyclophosphamide),[5][23][56][57] menstrual cycle-related mouth ulcers,[22] and as a component of menopausal hormone therapy.[40]
References
- 1 2 3 4 Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 338–. ISBN 978-3-7692-2114-5.
- ↑ Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 289–. ISBN 978-0-08-093292-7.
- 1 2 3 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 132–. ISBN 978-94-011-4439-1.
- 1 2 3 Breuer H, Lisboa BP (1966). "[Studies on the metabolism of 17-alpha-hydroxy-19-norprogesterone caproate by humans in vivo and of 17-alpha-hydroxy-19-norprogesterone by rats in vitro]". Acta Endocrinologica (in German). 51 (1): 114–30. PMID 4285463.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 Franz v. Bruchhausen; Gerd Dannhardt; Siegfried Ebel; August W. Frahm; Eberhard Hackenthal; Ulrike Holzgrabe (2 July 2013). Hagers Handbuch der Pharmazeutischen Praxis: Band 8: Stoffe E-O. Springer-Verlag. pp. 343–. ISBN 978-3-642-57994-3.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Die Gestagene. Springer-Verlag. 27 November 2013. pp. 6, 278–279. ISBN 978-3-642-99941-3.
- 1 2 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1761–1762. ISBN 978-0-8155-1856-3.
- ↑ David E. Thurston (22 November 2006). Chemistry and Pharmacology of Anticancer Drugs. CRC Press. pp. 154–155. ISBN 978-1-4200-0890-6.
- 1 2 G. Raspé (22 October 2013). Hormones and Embryonic Development: Advances in The Biosciences. Elsevier Science. p. 79. ISBN 978-1-4831-5171-7.
- 1 2 Schoonees, R.; De Klerk, J. N.; Murphy, G. P. (1969). "The effect of depostat (SH 582) on the baboon prostate". Journal of Surgical Oncology. 1 (4): 317–324. doi:10.1002/jso.2930010404. ISSN 0022-4790.
- 1 2 J. Horsky; J. Presl (6 December 2012). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 95–. ISBN 978-94-009-8195-9.
- 1 2 Schering A.G., Berlin (1968). Depostat (SH 582) a new treatment for prostatic hypertrophy.
- 1 2 3 4 Aubrey, D. A.; Khosla, T. (1971). "The effect of 17-alpha-hydroxy-19-norprogesterone caproate (SH 582) on benign prostatic hypertrophy". British Journal of Surgery. 58 (9): 648–652. doi:10.1002/bjs.1800580904. ISSN 0007-1323.
- 1 2 Kaiser, R. (1960). Klinische Erfahrungen mit Norprogesteronderivaten. Zbl. Gynäk, 82, 2009.
- 1 2 Subbiah, N.; Mortensen, James (1973). "The Treatment of Benign Enlargement of the Prostate with Nor Progesterone Caproate (Primostat)". ANZ Journal of Surgery. 42 (3): 304–307. doi:10.1111/j.1445-2197.1973.tb06805.x. ISSN 1445-1433.
- 1 2 3 4 5 6 7 8 9 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. p. 278. ISBN 978-3-88763-075-1.
- 1 2 3 4 5 https://www.drugs.com/international/gestonorone-caproate.html
- 1 2 3 4 5 6 http://www.micromedexsolutions.com/micromedex2/librarian/
- 1 2 3 4 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2105. ISBN 978-0-85369-840-1.
- ↑ H. John Smith; Hywel Williams (10 October 2005). Smith and Williams' Introduction to the Principles of Drug Design and Action, Fourth Edition. CRC Press. pp. 493–. ISBN 978-0-203-30415-0.
- 1 2 3 Aubrey DA, Khosla T (September 1971). "The effect of 17-alpha-hydroxy-19-norprogesterone caproate (SH582) on benign prostatic hypertrophy". Br J Surg. 58 (9): 648–52. doi:10.1002/bjs.1800580904. PMID 4105896.
- 1 2 3 Ferguson MM, McKay Hart D, Lindsay R, Stephen KW (October 1978). "Progeston therapy for menstrually related aphthae". Int J Oral Surg. 7 (5): 463–70. doi:10.1016/S0300-9785(78)80038-6. PMID 102602.
- 1 2 Ward HW (June 1972). "Progestogen therapy for ovarian carcinoma". J Obstet Gynaecol Br Commonw. 79 (6): 555–9. doi:10.1111/j.1471-0528.1972.tb14200.x. PMID 4555897.
- ↑ Karlstedt K (1971). "Progesterone treatment for local recurrence and metastases in carcinoma corporis uteri". Acta Radiologica: Therapy, Physics, Biology. 10 (2): 187–92. doi:10.3109/02841867109129755. PMID 5556820.
The preparations used were Proluton Depot (17a-hydroxy-progesterone caproate) and in 3 patients SH 5132 (17a-hydroxy-19-norprogesterone caproate); 100 mg of the latter corresponds to 1000 mg of Proluton Depot.
- ↑ Moe N (1972). "Short-term progestogen treatment of endometrial carcinoma. Histological, histochemical and hormonal studies". Acta Obstet Gynecol Scand. 51 (1): 55–62. doi:10.3109/00016347209154968. PMID 4261828.
Thirteen patients with primary adenocarcinoma of the uterine corpus were treated for 21 days with l7alphahydroxy-progesterone-caproate (Primolut Depot@, Schering), 1000 mg daily, or 17alpha-hydroxy-19-nor-progesterone-caproate (DepostatB, Schering), 200 mg daily. These doses can be considered as equivalent.
- ↑ G. Raspé; W. Brosig (22 October 2013). International Symposium on the Treatment of Carcinoma of the Prostate, Berlin, November 13 to 15, 1969: Life Science Monographs. Elsevier. p. 169. ISBN 978-1-4831-8711-2.
- ↑ Makrigiannis, D.; Gaca, A. (1971). "Evaluation of Depostat R in prostatic adenoma on the ground of clinical and sphincterotonometric studies". International Urology and Nephrology. 3 (1): 21–29. doi:10.1007/BF02081794. ISSN 0301-1623.
- 1 2 Sander S, Nissen-Meyer R, Aakvaag A (1978). "On gestagen treatment of advanced prostatic carcinoma". Scand. J. Urol. Nephrol. 12 (2): 119–21. doi:10.3109/00365597809179977. PMID 694436.
- ↑ Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clinical Endocrinology. 11 (5): 497–504. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881.
Another synthetic gestogen, 17-hydroxy-19-norprogesterone caproate (Depostat-Schering), 400 mg by i.m. weekly injections suppressed T levels to 25% of pretreatment values (Sander er al., 1978).
- ↑ Orestano F, Altwein JE (December 1976). "Testosterone metabolism in benign prostatic hypertrophy: in vivo studies of gestonorone caproate and cyproterone acetate". Br J Urol. 48 (6): 485–91. doi:10.1111/j.1464-410X.1976.tb06687.x. PMID 64267.
- ↑ Orestano F, Altwein JE, Knapstein P, Bandhauer K (June 1975). "Mode of action of progesterone, gestonorone capronate (Depostat) and cyproterone acetate (Androcur) on the metabolism of testosterone in human prostatic adenoma: in vitro and in vivo investigations". J. Steroid Biochem. 6 (6): 845–51. doi:10.1016/0022-4731(75)90313-1. PMID 1177428.
- ↑ Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ↑ Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ↑ Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ↑ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ↑ Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- ↑ Louis Denis (6 December 2012). The Medical Management of Prostate Cancer. Springer Science & Business Media. pp. 112–. ISBN 978-3-642-73238-6.
Gestonorone caproate, another progestational agent, was investigated at our institution. Eighteen patients with painful metastatic [prostate cancer] with objective relapse after orchiectomy were treated with 400 mg/week i.m.
- ↑ Benno Clemens Runnebaum; T. Rabe; L. Kiesel (6 December 2012). Future Aspects in Contraception: Proceeding of an International Symposium held in Heidelberg, 5–8 September 1984 Part 1 Male Contraception. Springer Science & Business Media. pp. 133–. ISBN 978-94-009-4910-2.
Gestonorone [caproate] 100 or 200 mg/week i.m.
- ↑ Palanca, Ernesto; Juco, Wilfrido (2008). "Conservative treatment of benign prostatic hyperplasia". Current Medical Research and Opinion. 4 (7): 513–520. doi:10.1185/03007997709109342. ISSN 0300-7995.
A study was carried out in 30 male patients with benign prostate hyperplasia to assess the effectiveness of treatment with a progestational agent, gestonorone caproate (200 mg), given intramuscularly every 7 days over a period of 2 to 3 months.
- 1 2 Lindsay R, Hart DM, Purdie D, Ferguson MM, Clark AS, Kraszewski A (February 1978). "Comparative effects of oestrogen and a progestogen on bone loss in postmenopausal women" (PDF). Clin Sci Mol Med. 54 (2): 193–5. doi:10.1042/cs0540193. PMID 340117.
- 1 2 3 Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- 1 2 3 4 5 6 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 595–. ISBN 978-1-4757-2085-3.
- ↑ Axel Kleemann; Jürgen Engel (2001). Pharmaceutical substances: syntheses, patents, applications. Thieme. p. 962. ISBN 978-3-13-558404-1.
- ↑ http://www.mhra.gov.uk/spc-pil/
- 1 2 Notter, G.; Berndt, G. (2009). "Hormonal Treatment of Mammary Carcinoma with Progynon-Depot and Depostat". Acta Radiologica: Therapy, Physics, Biology. 14 (5): 433–442. doi:10.3109/02841867509132684. ISSN 0567-8064.
- ↑ Ward, H. W. C. (1972). "PROGESTOGEN THERAPY FOR OVARIAN CARCINOMA". BJOG: An International Journal of Obstetrics and Gynaecology. 79 (6): 555–559. doi:10.1111/j.1471-0528.1972.tb14200.x. ISSN 1470-0328.
- ↑ Berndt, G.; Stender, H.-St. (2009). "[The combined estrogen-gestagen treatment of metastasizing mammary carcinoma using with SH 834]". 95 (48): 2399–2404. doi:10.1055/s-0028-1108843. ISSN 0012-0472.
- ↑ http://www.popline.org/node/485956
- ↑ Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94-95 https://www.popline.org/node/423289
- ↑ Dr. S. S. Kadam (July 2007). Principles of Medicinal Chemistry Volume 2. Pragati Books Pvt. Ltd. pp. 381–. ISBN 978-81-85790-03-9.
- ↑ Karim M, El-mahgoub S (September 1970). "Injectable steroids as a method of contraception". Ain Shams Med J. 21 (5): 543–50. PMID 12313080.
- ↑ Carlborg L (July 1973). "Effect of norhydroxyprogesterone caproate on cervical sperm penetration and secretion of ovarian steroids in the human female". Ups. J. Med. Sci. 78 (3): 189–90. doi:10.3109/03009737309178626. PMID 4797435.
- ↑ Hurtado, H; Kesseru, E; Larrañaga, Alfredo. "Empleo del capronato de 17-hidroxi-19-norprogesterona como anticonceptivo inyectable de depósito". Revista Peruana de Ginecología y Obstetricia. 14 (2): 223–233. doi:10.31403/rpgo.v14i1457.
- ↑ Nazer J, Valenzuela CY (March 1973). "[Possible biological effects of contraceptives]". Rev Med Chil (in Spanish; Castilian). 101 (3): 234–6. PMID 4732140.
- ↑ Rodriguez-Restrepo, R. (1969). 17-alpha-hydroxy 19 norprogesterone capronate as a prolonged-action injectable contraceptive agent. Revista Colombiana de Obstetricia y Ginecologia, 20, 247-255. https://www.popline.org/node/479231
- ↑ Guthrie D (July 1979). "The treatment of advanced cystadenocarcinoma of the ovary with gestronol and continuous oral cyclophosphamide". Br J Obstet Gynaecol. 86 (7): 497–500. doi:10.1111/j.1471-0528.1979.tb10799.x. PMID 476014.
- ↑ Darwish DH (August 1978). "The effect of sex steroids on the in vitro synthesis of DNA by malignant ovarian tumours". Br J Obstet Gynaecol. 85 (8): 627–33. doi:10.1111/j.1471-0528.1978.tb14933.x. PMID 687544.