Enobosarm
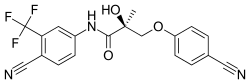 | |
| Clinical data | |
|---|---|
| Synonyms | GTx-024; MK-2866; Ostarine; S-22[1] |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 24 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C19H14F3N3O3 |
| Molar mass | 389.33 g/mol |
| 3D model (JSmol) | |
| Melting point | 132 to 136 °C (270 to 277 °F) |
| |
| |
| (verify) | |
Enobosarm, also known as ostarine, is an investigational selective androgen receptor modulator (SARM) developed by GTX, Inc for the treatment of conditions such as muscle wasting and osteoporosis, formerly under development by Merck & Company.
Chemistry
According to a 2009 paper authored by GTx, "Readers are cautioned to note that the name ostarine is often mistakenly linked to the chemical structure of [S-4], which is also known as andarine. The chemical structure of ostarine has not been publicly disclosed."[2] A 2009 review stated "Recently, GTx disclosed that compound 5 had advanced into clinical trials. The patent application described detailed data in an initial proof-of-concept Phase IIa clinical trial. It is not explicitly stated that compound 5 is Ostarine (MK-2866).[3]
History
GTx Incorporated was founded in Memphis in 1997 and licensed rights to enobosarm from the University of Tennessee Research Foundation; the SARM compounds from Tennessee had been invented by Karen Veverka and Michael Whitt and each later joined the company.[4]
By 2007 enobosarm was in a Phase II trial, and that year GTx signed an exclusive license agreement for its SARM program with Merck & Co.[5] The companies ended the deal in 2010.[6]
In August 2013 GTx announced that enobosarm had failed in two Phase III clinical trials to treat wasting in people with lung cancer.[7] The company had invested around $35 million in the development of the drug.[8] The company said at that time that is planned to pursue approval of enobosarm in Europe; the company was also still developing GTx-758 for castration-resistant prostate cancer.[9]
In 2016 GTx began Phase II trials, to see if enosobarm might be effective to treat stress uninary incontinence in women.[10]
Society and culture
Doping
SARMs including enobosarm may be and have been used by athletes to assist in training and increase physical stamina and fitness, potentially producing effects similar to anabolic steroids. For this reason, SARMs were banned by the World Anti-Doping Agency in January 2008 despite no drugs from this class yet being in clinical use, and blood tests for all known SARMs have been developed.[11][12] There are a variety of known cases of doping in sports with enobosarm by professional athletes.
In May 2017, Dynamic Technical Formulations voluntarily recalled all lots of Tri-Ton, a dietary supplement that the FDA tested and found to contain enobosarm and andarine.[13]
See also
References
- ↑ "Enobosarm - GTx". AdisInsight. Retrieved 25 April 2018.
- ↑ Mohler ML, Bohl CE, Jones A, et al. (June 2009). "Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit". J. Med. Chem. 52 (12): 3597–617. doi:10.1021/jm900280m. PMID 19432422.
- ↑ Zhang X, Lanter JC, Sui Z (September 2009). "Recent advances in the development of selective androgen receptor modulators". Expert Opin Ther Pat. 19 (9): 1239–58. doi:10.1517/13543770902994397. PMID 19505196. The first quoted sentence is cited to Published PCT application WO2008127717
- ↑ "GTX, INC. - FORM S-1/A". Gtx via SEC Edgar. 22 December 2003.
- ↑ Nagle, Mike (7 November 2007). "Merck flexes muscle with GTx deal". Outsourcing Pharma.
- ↑ Swanekamp, Kelsey (15 March 2010). "Merck And GTx Go Their Separate Ways". Forbes.
- ↑ "Enobosarm fails endpoints in Ph III study". The Pharma Letter. 20 August 2013.
- ↑ Sheffield, Michael (April 4, 2014). "Steiner resigns from GTx". Memphis Business Journal.
- ↑ Garde, Damian (4 April 2014). "GTx's CEO finds the door as the company moves on from a PhIII failure". FierceBiotech.
- ↑ "GTx begins Phase II trial of enobosarm to treat women with stress urinary incontinence - Drug Development Technology". Drug Development Technology. 14 January 2016.
- ↑ Thevis M, Kohler M, Schlörer N, Kamber M, Kühn A, Linscheid MW, Schänzer W. Mass spectrometry of hydantoin-derived selective androgen receptor modulators. Journal of Mass Spectrometry. 2008 May;43(5):639-50. PMID 18095383
- ↑ Thevis M, Kohler M, Thomas A, Maurer J, Schlörer N, Kamber M, Schänzer W. Determination of benzimidazole- and bicyclic hydantoin-derived selective androgen receptor antagonists and agonists in human urine using LC-MS/MS. Analytical and Bioanalytical Chemistry. 2008 May;391(1):251-61. PMID 18270691
- ↑ "Safety Alerts for Human Medical Products - Tri-Ton by Dynamic Technical Formulations, LLC: Recall - Contains Andarine and Ostarine Drug Ingredients". FDA. 22 May 2017.