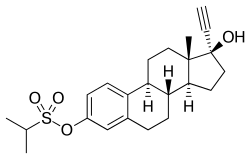
Ethinylestradiol sulfonate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Turisteron, others |
| Synonyms | EES; Turisteron; J96; Ethinylestradiol 3-isopropylsulfonate; Ethinylestradiol 3-(2-propanesulfonate); 17α-Ethynyl-3-isopropyl-sulfonyloxyestradiol |
| Routes of administration | By mouth[1][2] |
| Drug class | Estrogen; Estrogen ester |
| Pharmacokinetic data | |
| Metabolites | • Ethinylestradiol[1][2] |
| Elimination half-life | Oral: 6 days[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| Chemical and physical data | |
| Formula | C23H30O4S |
| Molar mass | 402.547 g/mol |
| 3D model (JSmol) | |
| |
| |
Ethinylestradiol sulfonate (EES), sold under the brand name Turisteron among others, is an estrogen medication which has been used in birth control in women and in the treatment of prostate cancer in men.[1][2][4] It has also been studied in the treatment of breast cancer in women.[3][5] The medication is taken by mouth once per week.[1][2]
Medical uses
EES has been used in combination with norethisterone acetate as a once-a-week birth control pill and as a high-dose estrogen monotherapy in the treatment of prostate cancer.[2][4][6] It has also been assessed in the treatment of breast cancer.[3][5] The drug is used at a dosage of 1 to 2 mg once per week in the treatment of prostate cancer.[1]
Side effects
EES has been described as having good tolerability compared to EE, and this property has been described as "remarkable".[1] The unique C3 sulfonate ester of EES seems to reduce its hepatic estrogenicity, which in turn reduces its adverse effects.[1] In particular, EES is said to have considerably reduced cardiovascular side effects relative to EE when used as a form of high-dose estrogen therapy in the treatment of prostate cancer.[1] This may in part be related to the greatly reduced oral dosing frequency of EES relative to EE, as parenteral EE, which bypasses the first pass through the liver that occurs with oral EE, has been found to have a 5-fold lower impact on liver protein synthesis than oral EE.[1]
Pharmacology
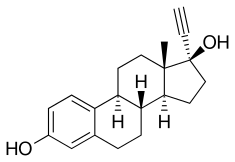
EES is an estrogen ester and long-acting prodrug of ethinylestradiol (EE) which is taken orally.[1][2] It is more lipophilic than EE, and this results in a depot effect in which EES is taken up into fat and then slowly released from it.[1] Following its release from fat, EES is hydrolyzed into EE.[1] As a result of this depot effect, EES has a very long biological half-life of about 6 days.[3][3] This allows it to be taken once per week.[2][1]
EES is a powerful antigonadotropin, and is capable of suppressing circulating testosterone concentrations to levels comparable to those seen with castration (less than 1 to 3% of initial values).[4][7][6] In addition, EE can strongly increase sex hormone-binding globulin (SHBG) levels, thereby additionally decreasing free testosterone levels.[8][9][7] As such, EES is a powerful functional antiandrogen, which makes it useful for treating prostate cancer.[10][7]
Chemistry
EES, also known as ethinylestradiol 3-isopropylsulfonate or ethinylestradiol 3-(2-propanesulfonate), is a synthetic estrane steroid and a derivative of estradiol. Specifically, it is the C3 isopropylsulfonate ester of ethinylestradiol (17α-ethynylestradiol).[11][12][1] EES is similar to quinestrol (EE 3-cyclopentyl ether), which is a C3 ether of EE and is a long-lasting oral depot estrogen similarly.[1]
Analogues of EES include ethinylestradiol N,N-diethylsulfamate (J271) and ethinylestradiol pyrrolidinosulfamate (J272).[2] These analogues are rapidly taken up by erythrocytes in the blood of the hepatic portal vein during the first pass with oral administration and have been found to be much stronger oral estrogens than EE or EES.[2] EE and EES themselves do not have affinity for erythrocytes.[2] EES and related C3 sulfur-containing esters of EE led to the development of estrogen sulfamates like estradiol 3-sulfamate (J995), estriol 3-sulfamate (J1034), and estradiol 17β-(1-(4-(aminosulfonyl)benzoyl)-L-proline) (EC508), which are highly potent oral estradiol prodrugs that bind to erythrocytes similarly and are under investigation for potential clinical use.[1][2][13][14]
History
EES was first synthesized around the late 1960s.[2] It appears to have been first introduced for medical use in the 1980s.[15]
Society and culture
Generic names
Ethinylestradiol sulfonate is the generic name of the drug, but it is also commonly known by its brand name Turisteron.[11][12] It does not appear to have an INN or other such designations.[11][12] EES has also been known by its former developmental code name J96.[2]
Brand names
EES has been marketed under the brand names Turisteron and Deposiston-Oestrogen.[12]
Availability
EES has been marketed in Germany, though it appears that it may no longer be available.[12][16][17]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 248, 277, 369, 540, 542. doi:10.1007/978-3-642-60107-1. ISBN 978-3-642-60107-1.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Elger W, Palme HJ, Schwarz S (April 1998). "Novel oestrogen sulfamates: a new approach to oral hormone therapy". Expert Opin Investig Drugs. 7 (4): 575–89. doi:10.1517/13543784.7.4.575. PMID 15991994.
- 1 2 3 4 5 Gürtler R, Tanneberger S, Bodek B, Morack G (1982). "[Clinical experience with the depot estrogen Turisteron in the treatment of metastatic breast cancer (author's transl)]". Arch. Geschwulstforsch. (in German). 52 (2): 129–39. PMID 7103689.
- 1 2 3 Dörner G, Schnorr D, Stahl F, Rohde W (December 1985). "Successful treatment of prostatic cancer with the orally active depot estrogen ethinylestradiol sulfonate (Turisteron)". Exp. Clin. Endocrinol. 86 (2): 190–6. doi:10.1055/s-0029-1210486. PMID 3912197.
- 1 2 S. Monfardini; K. Brunner; D. Crowther; S. Eckhardt; D. Olive; S. Tanneberger; A. Veronesi; J.M.A. Whitehouse; R. Wittes (1987). Manual of Adult and Paediatric Medical Oncology. Springer Science & Business Media. pp. 196–. doi:10.1007/978-3-642-82489-0. ISBN 978-3-642-82489-0.
- 1 2 Stahl F, Schnorr D, Bär CM, Fröhlich G, Dörner G (1989). "Suppression of plasma androgen levels with a combination therapy of depot-estrogen (Turisteron) and Dexamethasone in patients with prostatic cancer". Exp. Clin. Endocrinol. 94 (3): 239–43. doi:10.1055/s-0029-1210905. PMID 2630306.
- 1 2 3 Guddat HM, Schnorr D, Dörner G, Stahl F, Rohde W (December 1987). "[Behavior of LH, FSH, total testosterone, free testosterone and SHBG serum levels in the therapy of prostatic cancer with Turisteron (ethinyl estradiol sulfonate)]". Z Urol Nephrol (in German). 80 (12): 665–8. PMID 3126615.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 157–. ISBN 978-92-832-1291-1.
- ↑ Stephen J. Winters; Ilpo T. Huhtaniemi (25 April 2017). Male Hypogonadism: Basic, Clinical and Therapeutic Principles. Humana Press. pp. 307–. ISBN 978-3-319-53298-1.
- ↑ Schnorr D, Dörner G, Stahl F, Rohde W, Guddat HM (1987). "[Conservative therapy of prostate cancer using Turisteron]". Z Urol Nephrol (in German). 80 (3): 149–57. PMID 3111122.
- 1 2 3 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 523–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 412–. ISBN 978-3-88763-075-1.
- ↑ Elger W, Barth A, Hedden A, Reddersen G, Ritter P, Schneider B, Züchner J, Krahl E, Müller K, Oettel M, Schwarz S (2001). "Estrogen sulfamates: a new approach to oral estrogen therapy". Reprod. Fertil. Dev. 13 (4): 297–305. doi:10.1071/RD01029. PMID 11800168.
- ↑ Elger W, Wyrwa R, Ahmed G, Meece F, Nair HB, Santhamma B, Killeen Z, Schneider B, Meister R, Schubert H, Nickisch K (January 2017). "Estradiol prodrugs (EP) for efficient oral estrogen treatment and abolished effects on estrogen modulated liver functions". J. Steroid Biochem. Mol. Biol. 165 (Pt B): 305–311. doi:10.1016/j.jsbmb.2016.07.008. PMID 27449818.
- ↑ Vachalovský V, Vomácka V (November 1985). "[Turisteron in the treatment of advanced carcinoma of the prostate]". Rozhl Chir (in Czech). 64 (11): 718–22. PMID 4089702.
- ↑ Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2102. ISBN 978-0-85369-840-1.
- ↑ https://www.drugs.com/international/turisteron.html