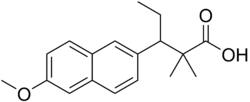
Methallenestril
 | |
| Clinical data | |
|---|---|
| Trade names | Cur-men, Ercostrol, Geklimon, Novestrine, Vallestril (also spelled Vallestrol or Vallestryl) |
| Synonyms | Methallenoestril; Methallenestrol; Methallenoestrol; Horeau's acid; Allenestrol 6-methyl ether; α,α-Dimethyl-β-ethylallenolic acid 6-methyl ether; β-Ethyl-6-methoxy-α,α-dimethyl-2-naphthalenepropionic acid |
| Routes of administration | By mouth |
| Drug class | Nonsteroidal estrogen |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ECHA InfoCard |
100.007.485 |
| Chemical and physical data | |
| Formula | C18H22O3 |
| Molar mass | 286.365 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Methallenestril (INN) (brand names Cur-men, Ercostrol, Geklimon, Novestrine, Vallestril), also known as methallenoestril (BAN) and as methallenestrol, as well as Horeau's acid,[1][2] is a synthetic nonsteroidal estrogen and a derivative of allenolic acid and allenestrol (specifically, a methyl ether of it) that was formerly used to treat menstrual issues but is now no longer marketed.[3][4][5][6] It is a seco-analogue of bisdehydrodoisynolic acid, and although methallenestril is potently estrogenic in rats, in humans it is only weakly so in comparison.[7] Vallestril was a brand of methallenestril issued by G. D. Searle & Company in the 1950s.[8] Methallenestril is taken by mouth.[9] By the oral route, a dose of 25 mg methallenestril is approximately equivalent to 1 mg diethylstilbestrol, 4 mg dienestrol, 20 mg hexestrol, 25 mg estrone, 2.5 mg conjugated estrogens, and 0.05 mg ethinylestradiol.[9]
See also
References
- ↑ Erich Heftmann (1970). Steroid Biochemistry. Academic Press. p. 144.
- ↑ Dodds, E. C. (1949). "Synthetic aestrogens". Journal of Pharmacy and Pharmacology. 1 (1): 137–147. doi:10.1111/j.2042-7158.1949.tb12391.x. ISSN 0022-3573.
- ↑ C.R. Ganellin; David J. Triggle (21 November 1996). Dictionary of Pharmacological Agents. CRC Press. pp. 1295–. ISBN 978-0-412-46630-4.
- ↑ I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 177–. ISBN 978-94-011-4439-1.
- ↑ John A. Thomas; Edward J. Keenan (1986). Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 171–. ISBN 978-1-4684-5036-1.
- ↑ Herbai G, Ljunghall S (1983). "Normalization of hypercalcaemia of primary hyperparathyroidism by treatment with methallenestril, a synthetic oestrogen with low oestrogenicity". Urol. Int. 38 (6): 371–3. doi:10.1159/000280925. PMID 6659184.
- ↑ Raymond Eller Kirk; Donald Frederick Othmer (1980). Encyclopedia of chemical technology. Wiley. p. 670. ISBN 978-0-471-02065-3.
- ↑ Library of Congress. Copyright Office (1965). Catalog of Copyright Entries. Third Series: 1963: July-December. Copyright Office, Library of Congress. pp. 1984–.
- 1 2 Swyer GI (April 1959). "The oestrogens" (PDF). Br Med J. 1 (5128): 1029–31. doi:10.1136/bmj.1.5128.1029. PMC 1993181. PMID 13638626.