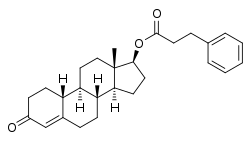
Nandrolone phenylpropionate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Durabolin, others |
| Synonyms |
• NPP • Nandrolone phenpropionate • 19-Nortestosterone phenylpropionate • Nandrolone hydrocinnamate • 19-Nortestosterone 17β-phenylpropionate • NSC-23162 |
| Pregnancy category | |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester; Progestogen |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
• Oral: 0.3–2.9% (pigs)[1] • Intramuscular: high[2] |
| Metabolism | Blood (hydrolysis), liver (reduction)[3][4] |
| Metabolites |
• Nandrolone[5] • 5α-Dihydronandrolone[5] • 19-Norandrosterone[6] • 19-Noretiocholanolone[6] • Conjugates[4] |
| Elimination half-life |
• Intramuscular: 2.7 days[7] • Nandrolone: <4.3 hours[3] |
| Duration of action | • Intramuscular: 5–7 days[5][7] |
| Excretion | Urine[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H34O3 |
| Molar mass | 406.57 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nandrolone phenylpropionate (NPP), or nandrolone phenpropionate, sold under the brand name Durabolin among others, is an androgen and anabolic steroid (AAS) medication which has been used primarily in the treatment of breast cancer and osteoporosis in women.[8][9][10][11][5] It is given by injection into muscle once every week.[5] Although it was widely used in the past, the drug has mostly been discontinued and hence is now mostly no longer available.[5][11]
Side effects of NPP include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[5] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[5][12] It has strong anabolic effects and weak androgenic effects, which give it a mild side effect profile and make it especially suitable for use in women and children.[5][12][13] NPP is a nandrolone ester and a long-lasting prodrug of nandrolone in the body.[5]
NPP was first described in 1957 and was introduced for medical use in 1959.[5] It was the first nandrolone ester to be introduced, followed by nandrolone decanoate in 1962, and has been one of the most widely used nandrolone esters.[5][14] However, in more recent times, the drug has been largely superseded by nandrolone decanoate, which is longer-acting and more convenient to use.[5][11] In addition to its medical use, NPP is used to improve physique and performance.[5] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[5]
Medical uses
NPP has been used mainly in the treatment of advanced breast cancer in women and as an adjunct therapy for the treatment of senile or postmenopausal osteoporosis in women.[5] Historically, it has also had a variety of other uses.[5] Because of its reduced androgenic effects, the drug has not generally been used in androgen replacement therapy for androgen deficiency in men and has instead been used for solely for anabolic indications.[2][15] However, nandrolone esters have more recently been proposed for the treatment of androgen deficiency in men due to favorable properties including their high ratio of anabolic to androgenic effects and consequent much lower risk of prostate enlargement, prostate cancer, and scalp hair loss relative to testosterone.[16][17]
Available forms
NPP is or has been available 25 mg/mL and 50 mg/mL formulations in oil for intramuscular injection.[5]
Non-medical uses
NPP is used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters.[5] Nandrolone esters have been said to be the most popular AAS used by bodybuilders and in sports.[5][18] This is in part due to the high ratio of anabolic to androgenic effect of nandrolone and its weak propensity for androgenic and estrogenic side effects.[5]
Side effects
The most common side effects of NPP consist of virilization (masculinization) in women, including symptoms such as acne, hirsutism (increased body/facial hair growth), hoarseness of the voice, and voice deepening.[5] However, relative to most other AAS, NPP has a greatly reduced propensity for virilization and such side effects are relatively uncommon at recommended dosages.[5] At higher dosages and/or with long-term treatment they make increase in incidence and magnitude however.[5] A variety of uncommon and rare side effects may also occur.[5]
Interactions
Antiestrogens like aromatase inhibitors (e.g., anastrozole) and selective estrogen receptor modulators (e.g., tamoxifen, raloxifene) can interfere with and prevent the estrogenic effects of NPP.[5] 5α-Reductase inhibitors like finasteride and dutasteride can prevent the inactivation of nandrolone in so-called "androgenic" tissues like the skin, hair follicles, and prostate gland and may therefore considerably increase its androgenic side effects.[5] This is opposite to the case of most other AAS, which are either potentiated by 5α-reductase in such tissues or are not metabolized by 5α-reductase.[5] Antiandrogens like cyproterone acetate, spironolactone, and bicalutamide can block both the anabolic and androgenic effects of NPP.[19]
Pharmacology
Pharmacodynamics
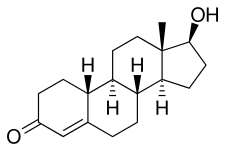
NPP is a nandrolone ester, or a prodrug of nandrolone.[20][5] As such, it is an androgen and anabolic steroid, or an agonist of the androgen receptor, the biological target of androgens like testosterone.[5][20] Relative to testosterone, NPP has enhanced anabolic effects and reduced androgenic effects.[20][5] In addition to its anabolic and androgenic activity, NPP has low estrogenic activity (via its metabolite estradiol) and moderate progestogenic activity.[5] Like other AAS, NPP has antigonadotropic effects, which are due to both its androgenic and progestogenic activity.[5]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Nandrolone | 20 | 154–155 | <0.1 | 0.5 | 1.6 | 1–16 | 0.1 |
| Testosterone | 1.0–1.2 | 100 | <0.1 | 0.17 | 0.9 | 19–82 | 3–8 |
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 |
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. a = 1-hour incubation time (4 hours is standard for this assay; may affect affinity value). Sources:[21][22][23][24][25][26][27][28] | |||||||
| Compound | rAR (%) | hAR (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Testosterone | 38 | 38 | ||||||
| 5α-Dihydrotestosterone | 77 | 100 | ||||||
| Nandrolone | 75 | 92 | ||||||
| 5α-Dihydronandrolone | 35 | 50 | ||||||
| Ethylestrenol | ND | 2 | ||||||
| Norethandrolone | ND | 22 | ||||||
| 5α-Dihydronorethandrolone | ND | 14 | ||||||
| Metribolone | 100 | 110 | ||||||
| Abbreviations: rAR = Rat prostate androgen receptor at 4°C. hAR = Intact human MCF-7 breast cancer androgen receptor at 37°C. Miscellaneous: Direct link to table. Sources: [29][30] | ||||||||
Pharmacokinetics

NPP is converted into nandrolone in the body, which is the active form of the drug.[5] It has an extended elimination half-life in the body when administered via intramuscular injection.[20] Its duration of action is approximately one week and it is administered once every few days to once per week.[5] The elimination half-life and duration of action of NPP are much shorter than those of nandrolone decanoate.[5][31]
Chemistry
Nandrolone phenylpropionate, or nandrolone 17β-phenylpropionate, is a synthetic estrane steroid and a derivative of testosterone.[8][9] It is an androgen ester; specifically, it is the C17β phenylpropionate ester of nandrolone (19-nortestosterone), which itself is the 19-demethylated analogue of testosterone.[8][9]
History
NPP was first described in 1957 and was introduced for medical use in 1959.[5][32] It was initially used for a wide variety of indications, but starting in the 1970s its use became more restricted and its main uses became the treatment of breast cancer and osteoporosis in women.[5] Today, NPP is scarcely available.[5] The drug was the first form of nandrolone to be introduced, and was followed by nandrolone decanoate in 1962, which has been more widely used in comparison.[32]
Society and culture
Generic names
Nandrolone phenylpropionate is the generic name of the drug and its BAN while nandrolone phenpropionate is its USAN.[8][9][10][11] It has also been referred to as nandrolone phenylpropanoate or as nandrolone hydrocinnamate.[8][9][10][11]
Brand names
NPP is or has been marketed under a variety of brand names including Durabolin, Fenobolin, Activin, Deca-Durabolin, Evabolin, Grothic, Hybolin Improved, Metabol, Nerobolil, Neurabol, Norabol, Noralone, Sintabolin, Strabolene, Superanabolon, and Turinabol.[8][9][10][11]
Availability
NPP is or has been marketed in many countries throughout the world, including in the United States, the United Kingdom, and Canada.[9][11]
United States
NPP was marketed previously in the United States but is no longer available in this country.[33] Nandrolone decanoate, conversely, is one of the few AAS that remains available for medical use in this country.[33]
Legal status
NPP, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.[34]
References
- ↑ McEvoy JD, McVeigh CE, McCaughey WJ (1998). "Residues of nortestosterone esters at injection sites. Part 1. Oral bioavailability". Analyst. 123 (12): 2475–8. doi:10.1039/a804919j. PMID 10435281.
- 1 2 Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1185–. ISBN 978-0-7817-1750-2.
- 1 2 3 http://www.medsafe.govt.nz/profs/Datasheet/d/Decadurabolininj.pdf
- 1 2 John A. Thomas (6 December 2012). Drugs, Athletes, and Physical Performance. Springer Science & Business Media. pp. 27–29. ISBN 978-1-4684-5499-4.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 460–467, 193–194. ISBN 978-0-9828280-1-4.
- 1 2 Victor A. Rogozkin (14 June 1991). Metabolism of Anabolic-Androgenic Steroids. CRC Press. pp. 108–. ISBN 978-0-8493-6415-0.
- 1 2 3 Minto CF, Howe C, Wishart S, Conway AJ, Handelsman DJ (1997). "Pharmacokinetics and pharmacodynamics of nandrolone esters in oil vehicle: effects of ester, injection site and injection volume". J. Pharmacol. Exp. Ther. 281 (1): 93–102. PMID 9103484.
- 1 2 3 4 5 6 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 660–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 716–717. ISBN 978-3-88763-075-1.
- 1 2 3 4 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- 1 2 3 4 5 6 7 https://www.drugs.com/international/nandrolone.html
- 1 2 Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ↑ Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 401–. ISBN 978-3-642-66353-6.
- ↑ Walter Sneader (23 June 2005). Drug Discovery: A History. John Wiley & Sons. pp. 206–. ISBN 978-0-471-89979-2.
- ↑ A. Wayne Meikle (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 271–. ISBN 978-1-59259-700-0.
- ↑ Wu C, Kovac JR (2016). "Novel Uses for the Anabolic Androgenic Steroids Nandrolone and Oxandrolone in the Management of Male Health". Curr Urol Rep. 17 (10): 72. doi:10.1007/s11934-016-0629-8. PMID 27535042.
- ↑ Pan MM, Kovac JR (2016). "Beyond testosterone cypionate: evidence behind the use of nandrolone in male health and wellness". Transl Androl Urol. 5 (2): 213–9. doi:10.21037/tau.2016.03.03. PMC 4837307. PMID 27141449.
- ↑ J. Larry Jameson; Leslie J. De Groot (25 February 2015). Endocrinology: Adult and Pediatric E-Book. Elsevier Health Sciences. pp. 2388–. ISBN 978-0-323-32195-2.
- ↑ Carrie Bagatell; William J. Bremner (27 May 2003). Androgens in Health and Disease. Springer Science & Business Media. pp. 25–. ISBN 978-1-59259-388-0.
- 1 2 3 4 Gao W, Bohl CE, Dalton JT (2005). "Chemistry and structural biology of androgen receptor". Chem. Rev. 105 (9): 3352–70. doi:10.1021/cr020456u. PMC 2096617. PMID 16159155.
- ↑ Delettré J, Mornon JP, Lepicard G, Ojasoo T, Raynaud JP (January 1980). "Steroid flexibility and receptor specificity". J. Steroid Biochem. 13 (1): 45–59. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
- ↑ Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ↑ Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". J. Steroid Biochem. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- ↑ Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Res. 38 (11 Pt 2): 4186–98. PMID 359134.
- ↑ Ojasoo T, Raynaud JP, Doé JC (January 1994). "Affiliations among steroid receptors as revealed by multivariate analysis of steroid binding data". J. Steroid Biochem. Mol. Biol. 48 (1): 31–46. doi:10.1016/0960-0760(94)90248-8. PMID 8136304.
- ↑ Saartok T, Dahlberg E, Gustafsson JA (June 1984). "Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin". Endocrinology. 114 (6): 2100–6. doi:10.1210/endo-114-6-2100. PMID 6539197.
- ↑ Cunningham GR, Tindall DJ, Lobl TJ, Campbell JA, Means AR (September 1981). "Steroid structural requirements for high affinity binding to human sex steroid binding protein (SBP)". Steroids. 38 (3): 243–62. doi:10.1016/0039-128X(81)90061-1. PMID 7197818.
- ↑ Pugeat MM, Dunn JF, Nisula BC (July 1981). "Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 69–75. doi:10.1210/jcem-53-1-69. PMID 7195405.
- ↑ Bergink EW, Janssen PS, Turpijn EW, van der Vies J (June 1985). "Comparison of the receptor binding properties of nandrolone and testosterone under in vitro and in vivo conditions". J. Steroid Biochem. 22 (6): 831–6. doi:10.1016/0022-4731(85)90293-6. PMID 4021486.
- ↑ Bergink EW, Geelen JA, Turpijn EW (1985). "Metabolism and receptor binding of nandrolone and testosterone under in vitro and in vivo conditions". Acta Endocrinol Suppl (Copenh). 271: 31–7. doi:10.1530/acta.0.109S0031. PMID 3865479.
- ↑ Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1362–. ISBN 978-1-60913-345-0.
- 1 2 Consolidated List of Products Whose Consumption And/or Sale Have Been Banned, Withdrawn, Severely Restricted Or Not Approved by Governments. United Nations Publications. 1983. pp. 153–154. ISBN 978-92-1-130230-1.
- 1 2 "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 17 December 2016.
- ↑ Steven B. Karch, MD, FFFLM (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
Further reading
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Wu C, Kovac JR (2016). "Novel Uses for the Anabolic Androgenic Steroids Nandrolone and Oxandrolone in the Management of Male Health". Curr Urol Rep. 17 (10): 72. doi:10.1007/s11934-016-0629-8. PMID 27535042.
- Pan MM, Kovac JR (2016). "Beyond testosterone cypionate: evidence behind the use of nandrolone in male health and wellness". Transl Androl Urol. 5 (2): 213–9. doi:10.21037/tau.2016.03.03. PMC 4837307. PMID 27141449.
External links