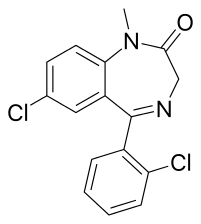
Diclazepam
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, sublingual |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Hepatic |
| Elimination half-life | ~42 hours[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C16H12Cl2N2O |
| Molar mass | 319.185 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Diclazepam (Ro5-3448), also known as chlorodiazepam and 2'-chloro-diazepam, is a benzodiazepine and functional analog of diazepam. It was first synthesized by Leo Sternbach and his team at Hoffman-La Roche in 1960.[2] It is not currently approved for use as a medication, but rather sold as an unscheduled substance.[3][4][5] Efficacy and safety have not been tested in humans.
In animal models, its effects are similar to diazepam, possessing long-acting anxiolytic, anticonvulsant, hypnotic, sedative, skeletal muscle relaxant, and amnestic properties.
Metabolism
Metabolism of this compound has been assessed,[1] revealing diclazepam has an approximate elimination half-life of 42 hours and undergoes N-demethylation to delorazepam, which can be detected in urine for 6 days following administration of the parent compound.[6] Other metabolites detected were lorazepam and lormetazepam which were detectable in urine for 19 and 11 days, respectively, indicating hydroxylation by cytochrome P450 enzymes occurring concurrently with N-demethylation.
Legal status
United Kingdom
In the UK, diclazepam has been classified as a Class C drug by the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other benzodiazepine drugs.[7]
See also
- Benzodiazepine
- Diazepam
- Delorazepam (Nordiclazepam)
- Lorazepam
- Phenazepam
- Ro5-4864 (4'-Chlorodiazepam)
References
- 1 2 Moosmann B, Bisel P, Auwärter V (July–August 2014). "Characterization of the designer benzodiazepine diclazepam and preliminary data on its metabolism and pharmacokinetics". Drug Testing and Analysis. 6 (7–8): 757–63. doi:10.1002/dta.1628. PMID 24604775.
- ↑ US 3136815, "Amino substituted benzophenone oximes and derivatives thereof"
- ↑ Madeleine Pettersson Bergstrand; Anders Helander; Therese Hansson; Olof Beck (2016). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Testing and Analysis. doi:10.1002/dta.2003. PMID 27366870.
- ↑ Høiseth, Gudrun; Tuv, Silja Skogstad; Karinen, Ritva (2016). "Blood concentrations of new designer benzodiazepines in forensic cases". Forensic Science International. 268: 35–38. doi:10.1016/j.forsciint.2016.09.006. PMID 27685473.
- ↑ Manchester, Kieran R.; Maskell, Peter D.; Waters, Laura (2018). "Experimental versus theoretical log D7.4, pKa and plasma protein binding values for benzodiazepines appearing as new psychoactive substances". Drug Testing and Analysis. doi:10.1002/dta.2387. ISSN 1942-7611. PMID 29582576.
- ↑ Bareggi SR, Truci G, Leva S, Zecca L, Pirola R, Smirne S (1988). "Pharmacokinetics and bioavailability of intravenous and oral chlordesmethyldiazepam in humans". European Journal of Clinical Pharmacology. 34 (1): 109–112. doi:10.1007/bf01061430. PMID 2896126.
- ↑ "The Misuse of Drugs Act 1971 (Amendment) Order 2017".