Polyestradiol phosphate
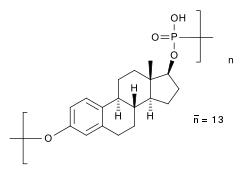 Polyestradiol phosphate structure | |
 Structure of estradiol phosphate, similar to one repeat unit of polyestradiol phosphate | |
| Clinical data | |
|---|---|
| Trade names | Estradurin, Estradurine |
| Synonyms | PEP; Estradiol phosphate polymer; Estradiol 17β-phosphate polymer; Estradiol polymer with phosphoric acid; Leo-114 |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection[1][2] |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High |
| Protein binding | >95% (estradiol)[1] |
| Metabolism | Mainly in the liver, to a lesser extent in the kidneys, gonads, and muscle (by phosphatases)[1] |
| Metabolites | Estradiol, phosphoric acid, and metabolites of estradiol[3][4] |
| Elimination half-life |
PEP: 70 days (10 weeks)[5] Estradiol: 1–2 hours[6] |
| Excretion | Urine (as conjugates)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem SID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula |
(C18H23O4P)n (n = variable; n = 13) |
| Molar mass |
Polymer: Variable Repeat unit: 334.347 g/mol |
| Melting point | 195 to 202 °C (383 to 396 °F) |
| (verify) | |
Polyestradiol phosphate (PEP), sold under the brand name Estradurin, is a medication which is used primarily in the treatment of prostate cancer in men.[1][2][7][8][9] It is also used in women to treat breast cancer and as a component of hormone therapy to treat low estrogen levels and menopausal symptoms.[1] It is given by injection into muscle once every four weeks.[1][2][10]
Common side effects of PEP include headache, breast tenderness, breast development, feminization, sexual dysfunction, infertility, and vaginal bleeding.[1][2] PEP is a synthetic estrogen and hence is an agonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[2][4][3] It is an estrogen ester in the form of a polymer and is an extremely long-lasting prodrug of estradiol in the body.[2][10][3][4] Because it works by being converted into estradiol, PEP is considered to be a natural and bioidentical form of estrogen.[3][2] The safety profile of parenteral estradiol esters like PEP is greatly improved relative to synthetic oral estrogens like ethinylestradiol and diethylstilbestrol.[2]
PEP was discovered around 1953 and was introduced for medical use in the United States in 1957.[10][11][12] Along with estradiol undecylate and estradiol valerate, it has been frequently used in the United States and Europe as a parenteral form of estrogen to treat men with prostate cancer.[8] However, it is no longer available in the United States.[11][13]
Medical uses
PEP is used as an intramuscular injection for estrogen therapy of prostate cancer in men.[1][2] It is also used to treat breast cancer in women who are at least 5 years postmenopausal.[1] In addition, PEP is used in hormone replacement therapy for low estrogen levels due to hypogonadism or menopause in women.[1] PEP is a form of high-dose estrogen therapy.[2] After an injection, it very slowly releases the active agent estradiol over at least several months.[14][5]
PEP has been compared to combined androgen blockade (CAB; castration plus flutamide) for the treatment of prostate cancer in a large randomized clinical trial of 915 patients.[15][16] At 18.5 months, there was no difference in survival or cardiovascular toxicity between the two treatment modalities.[15][16] These findings suggest that parenteral forms of estradiol may have similar effectiveness and safety relative to androgen deprivation therapy (ADT) in the treatment of prostate cancer.[15][16] In addition, estrogens may have significant advantages relative to ADT in terms of bone loss and fractures, hot flashes, sexual function, and quality of life, as well as considerable cost savings with parenteral forms of estradiol compared to GnRH analogue therapy.[15][16] On the other hand, breast tenderness and gynecomastia occur at very high rates with estrogens, whereas incidences are low with castration and CAB.[17] However, gynecomastia with estrogens is generally only mild-to-moderate in severity and is usually only modestly discomforting.[2] In addition, gynecomastia caused by estrogens can be prevented with prophylactic irradiation of the breasts or can be remediated with mastectomy.[2]
For prostate cancer in men, PEP is usually given at a dosage of 80 to 320 mg every 4 weeks for the first 2 to 3 months to rapidly build up estradiol levels.[1] Thereafter, to maintain estradiol levels, the dosage is adjusted down usually to 40 to 160 mg every 4 weeks based on clinical findings and laboratory parameters.[1] For breast cancer and low estrogen levels in women, the dosage is 40 to 80 mg every 4 weeks.[1]
Available forms
PEP is available in the form of vials and ampoules alone or in combination with mepivacaine and/or nicotinamide (vitamin B3) for administration via intramuscular injection.[1][18] Mepivacaine is a local anaesthetic and is used to avoid a burning sensation during injection of PEP.[1]
Contraindications
The contraindications of PEP are mostly the same as those of estradiol and include:[1]
- Hypersensitivity to PEP, mepivacaine, or other ingredients
- Known, previous, or suspected breast cancer or other estrogen-dependent malignant tumors (e.g., endometrial cancer)
- Vaginal bleeding of unknown cause or untreated endometrial hyperplasia
- Thrombosis and related, including active thrombophlebitis, former or current venous thromboembolism (deep vein thrombosis, pulmonary embolism), active or recent arterial thromboembolism (e.g., angina, myocardial infarction), or known thrombophilia (e.g., protein C deficiency, protein S deficiency, antithrombin deficiency)
- Severe arrhythmia, hypotension, hypertension, or lipid metabolism disorders
- Cerebrovascular events (i.e., stroke)
- Acute liver disease or previously confirmed liver disease, with abnormal liver function tests or jaundice (e.g., Dubin-Johnson syndrome, Rotor syndrome)
- Severe hepatic dysfunction
- Others including porphyria, sickle cell anemia, otosclerosis, or myasthenia gravis
- Pregnancy, lactation, and breastfeeding
Side effects
Systematic studies of the side effects of PEP are lacking.[1] However, its side effects are assumed to be identical to those of estradiol and other estradiol esters.[1] The side effects of PEP are partially dependent on sex.[1] Common or frequent (>10%) side effects are considered to include headache, abdominal pain, nausea, rash, pruritus, loss of libido, erectile dysfunction, breast tenderness, gynecomastia, feminization, demasculinization, infertility, and vaginal bleeding or spotting.[1][19] Side effects that occur occasionally or uncommonly (0.1–1%) include sodium and water retention, edema, hypersensitivity, breast tension, depression, dizziness, visual disturbances, palpitations, dyspepsia, erythema nodosum, urticaria, and chest pain.[1] All other side effects of PEP are considered to be rare.[1]
The rare (<0.1%) side effects of PEP are considered to include weight gain, impaired glucose tolerance, mood changes (elation or depression), nervousness, tiredness, headache, migraine, intolerance of contact lenses, hypertension, thrombosis, thrombophlebitis, thromboembolism, heart failure, myocardial infarction, vomiting, bloating, cholestatic jaundice, cholelithiasis, transient increases in transaminases and bilirubin, erythema multiforme, hyperpigmentation, muscle cramps, dysmenorrhea, vaginal discharge, premenstrual-like symptoms, breast enlargement, testicular atrophy, allergic reactions (e.g., urticaria, bronchial asthma, anaphylactic shock) due to mepivacaine, and injection site reactions (e.g., pain, sterile abscesses, inflammatory infiltrates).[1]
As thromboembolic and other cardiovascular complications are associated mainly with synthetic oral estrogens like ethinylestradiol and diethylstilbestrol, they occur much less often with parenteral bioidentical forms of estrogen like PEP.[1][2]
Cardiovascular effects
PEP does not produce undesirable effects on coagulation factors and is not believed to increase the risk of blood clots.[20][21] This is in spite of the fact that estradiol levels can reach high concentrations of as much as 700 pg/mL with high-dose PEP therapy.[22] It is also in contrast to oral synthetic estrogens such as diethylstilbestrol and ethinylestradiol, which produce marked increases in coagulation factors and high rates of blood clots at the high doses used to achieve castrate levels of testosterone in prostate cancer.[20][21][4] The difference between the two types of therapies is due to the bioidentical and parenteral nature of PEP and its minimal influence on liver protein synthesis.[20][21][4] PEP might actually reduce the risk of blood clots, due to decreases in levels of certain procoagulatory proteins.[20][21]
Small early pilot studies of PEP for prostate cancer in men found no cardiovascular toxicity with the therapy.[20] However, PEP has subsequently been found in large studies to significantly increase cardiovascular morbidity relative to GnRH modulators and orchiectomy in men treated with it for prostate cancer.[20][21] The increase in cardiovascular morbidity with PEP therapy is due to an increase in non-fatal ischemic heart events and heart decompensation.[23][24] Conversely, PEP has not been found to significantly increase cardiovascular mortality relative to GnRH modulators and orchiectomy.[20][21] Some studies have found that the increased cardiovascular morbidity with PEP is confined mainly to the first one or two years of therapy, whereas one study found consistently increased cardiovascular morbidity across three years of therapy.[20] A longitudinal risk analysis that projected over 10 years suggested that the cardiovascular risks of PEP may be reversed with long-term treatment and that the therapy may eventually result in significantly decreased cardiovascular risk relative to GnRH modulators and orchiectomy, although this has not been confirmed.[20]
The cardiovascular toxicity of PEP is far less than that of oral synthetic estrogens like diethylstilbestrol and ethinylestradiol, which increase the risk of venous and arterial thromboembolism, consequently increase the risk of transient ischemic attack, cerebrovascular accident (stroke), and myocardial infarction (heart attack), and result in substantial increases in cardiovascular mortality.[20][21] It is thought that the relatively minimal cardiovascular toxicity of parenteral forms of estradiol, like PEP and high-dose transdermal estradiol patches,[25] is due to their absence of effect on hepatic coagulation factors.[20][21]
Overdose
Acute toxicity studies have not indicated a risk of acute side effects with overdose of PEP.[1] The most likely sign of overdose is reversible feminization, namely gynecomastia.[1] There is no specific antidote for overdose of PEP, and treatment should be symptomatic.[1]
Interactions
Known potential interactions of PEP are mostly the same as those of estradiol and include:[1]
- Cytochrome P450 inhibitors, especially of CYP3A4, can reduce the metabolism of estradiol and thereby increase estradiol levels; examples include anti-infectives (e.g., erythromycin, clarithromycin, ketoconazole, itraconazole), cimetidine, and grapefruit juice[1][26]
- Cytochrome P450 inducers, especially of CYP3A4, can induce the metabolism of estradiol and thereby decrease estradiol levels; examples include anticonvulsants (e.g., phenobarbital, carbamazepine, phenytoin), anti-infectives (rifampicin, rifabutin, nevirapine, and efavirenz), and St. John's wort; in addition, while ritonavir and nelfinavir are known as strong inhibitors, they have an inducing effect in combination with steroid hormones[1]
- Certain antibiotics (e.g., ampicillin, tetracyclines) may decrease estradiol levels by limiting enterohepatic recirculation of estradiol[1]
- Paracetamol (acetaminophen), certain beta blockers (e.g., metoprolol), and some benzodiazepines may increase the effects of PEP[1]
- The coagulation-promoting effects of PEP may enhance those of aminocaproic acid[1]
- Polystyrene phosphate can reduce the effects of anticoagulants[1]
- Estrogens increase thyroxine-binding globulin levels and may result in increased thyroid hormone levels; interpretation of thyroid function tests should consider this[1]
- Due to reduced glucose tolerance, there may be an influence on need for insulin or oral antidiabetic medications[1]
- PEP may impair the effects of fibrates (e.g., bezafibrate) and certain nonsteroidal anti-inflammatory drugs (e.g., phenazone)[1]
- Concurrent use of hepatotoxic medications, especially dantrolene, may increase the risk of hepatotoxicity[1]
- Phosphatase inhibitors like levamisole may inhibit the cleavage of PEP into estradiol
Interactions with PEP may be less than with oral estrogens due to the lack of the first-pass through the liver.[1]
Pharmacology
Pharmacodynamics
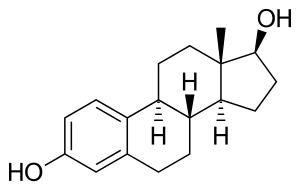
PEP is an estradiol ester in the form of a polymer and is an extremely long-lasting prodrug of estradiol.[2][5][3][4] As such, it is an estrogen, or an agonist of the estrogen receptors.[2][4][3] PEP has antigonadotropic and functional antiandrogenic effects due to its estrogenic activity.[22] A single repeat unit of PEP, corresponding to estradiol phosphate (minus OH2), is of about 23% higher molecular weight than estradiol due to the presence of its C17β phosphate ester.[27][8] Because PEP is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[3]
| Estrogen | Type | EPD (14 days) | Duration | |
|---|---|---|---|---|
| Estradiol benzoate | Bioidentical | 25–30 mg | 5 mg ≈ 5 days | |
| Estradiol dipropionate | Bioidentical | 25–30 mg | 5 mg ≈ 5–8 days | |
| Estradiol valerate | Bioidentical | 20 mg | 10 mg ≈ 14 days | |
| Estradiol cypionate | Bioidentical | 25–30 mg | 5 mg ≈ 14 days | |
| Polyestradiol phosphate | Bioidentical | 40–60 mg | 40 mg ≈ 28 days | |
| Diethylstilbestrol | Synthetic | 20 mg | 3 mg ≈ 3 days | |
| Diethylstilbestrol dipropionate | Synthetic | 15 mg | 2.5 mg ≈ 5 days | |
| Addendum: An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month.[28] Notes: All of the estrogens are by intramuscular injection. Abbreviations: EPD = Endometrial proliferation dose. Miscellaneous: Direct link to table. Sources: [29][30] | ||||
Antigonadotropic effects
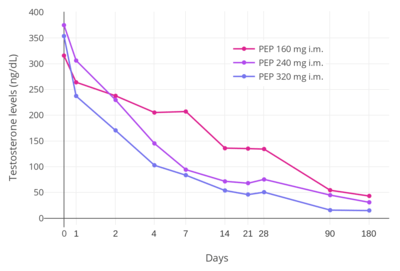
PEP has powerful antigonadotropic effects due to its estrogenic activity.[23] It has been found to suppress testosterone levels in men by 55%, 75%, and 85% at intramuscular dosages of 80, 160, and 240 mg every 4 weeks, respectively.[31] A single intramuscular injection of 320 mg PEP in men has been found to suppress testosterone levels to within the castrate range (< 50 ng/dL) within 3 weeks.[5] This was associated with circulating estradiol levels of just over 200 pg/mL.[22] The suppression of testosterone levels that can be achieved with PEP is equal to that with orchiectomy.[32] However, to achieve such concentrations of testosterone, which are about 15 ng/dL on average, higher concentrations of estradiol of around 500 pg/mL were necessary.[22][32][33] This was associated with a dosage of intramuscular 320 mg PEP every four weeks and occurred by 90 days of treatment.[22]
Mechanism of action in prostate cancer
The growth of prostate cancer is generally stimulated by dihydrotestosterone (DHT), and unless the cancer is castration-resistant, it can be treated by depriving it of androgens. Estradiol produces its therapeutic benefits mainly via exertion of negative feedback on the hypothalamic–pituitary–gonadal axis.[23][31][5] This blocks the secretion of luteinizing hormone, which in turn reduces testosterone production in the Leydig cells of the testes.[23][31][5] Estradiol also decreases the free percentage of testosterone by increasing sex hormone-binding globulin (SHBG) levels.[5] In addition, it exhibits direct cytotoxicity on prostate cancer cells.[34][14]
Differences from other estrogens
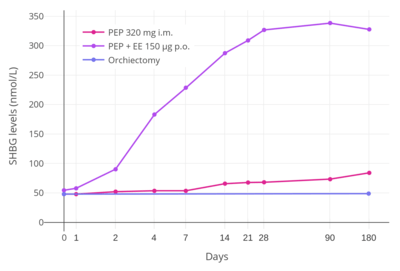
Estrogens have effects on liver protein synthesis, including on the synthesis of plasma proteins, coagulation factors, lipoproteins, and triglycerides.[32] These effects can result in an increased risk of thromboembolic and cardiovascular complications, which in turn can result in increased mortality.[32] Studies have found a markedly increased 5-year risk of cardiovascular mortality of 14 to 26% in men treated with oral synthetic estrogens like ethinylestradiol and diethylstilbestrol for prostate cancer.[32] However, whereas oral synthetic estrogens have a strong influence on liver protein synthesis, the effects of parenteral bioidentical estrogens like PEP on liver protein synthesis are comparatively very weak or even completely abolished.[32] This is because the first-pass through the liver with oral administration is avoided and because bioidentical estrogens are efficiently inactivated in the liver.[32]
A study found that whereas 320 mg/month intramuscular PEP increased SHBG levels to 166% in men with prostate cancer, the combination of 80 mg/month intramuscular polyestradiol phosphate and 150 µg/day oral ethinylestradiol increased levels of SHBG to 617%, an almost 8-fold difference in increase and almost 4-fold difference in absolute levels between the two treatment regimens.[22][5][35] In addition, whereas there were no cardiovascular complications in the PEP-only group, there was a 25% incidence of cardiovascular complications over the course of a year in the group that was also treated with ethinylestradiol.[5] Another study found no change in levels of coagulation factor VII, a protein of particular importance in the cardiovascular side effects of estrogens, with 240 mg/month intramuscular PEP.[36] These findings demonstrate the enormous impact of synthetic oral estrogens like ethinylestradiol on liver protein production relative to parenteral bioidentical forms of estrogen like PEP.[5]
Pharmacokinetics
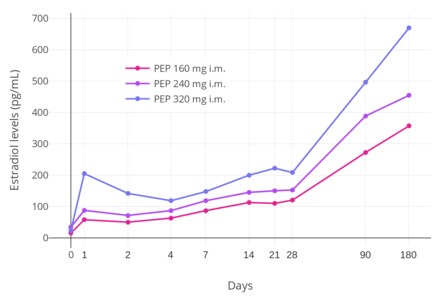
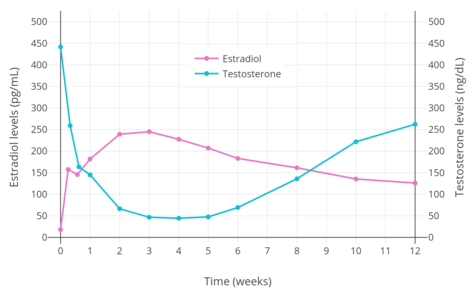
PEP reaches the bloodstream within hours after an injection (90% after 24 hours), where it circulates, and is accumulated in the reticuloendothelial system.[34] Estradiol is then cleaved from the polymer by phosphatases, although very slowly.[37] Levels of estradiol in men with intramuscular injections of PEP once every 4 weeks were about 350 pg/mL with 160 mg, 450 pg/mL with 240 mg, and almost 700 pg/mL with 320 mg, all measured after 6 months of treatment.[22] With monthly injections, steady-state estradiol concentrations are reached after 6 to 12 months.[34] Estradiol is metabolized primarily in the liver by CYP3A4 and other cytochrome P450 enzymes, and is metabolized to a lesser extent in extrahepatic tissues.[14][1] The metabolites are mainly excreted in urine via the kidneys.[1]
PEP has a very long duration and is given by intramuscular injection once every 4 weeks.[22] In men, an initial intramuscular injection of PEP results in a rapid rise in estradiol levels measured at 24 hours followed by a slow and gradual further increase in levels up until at least day 28 (the time of the next injection).[22] Subsequent injections result in a progressive and considerable accumulation in estradiol levels up to at least 6 months.[22] The mean terminal half-life of PEP has been found to be 70 days (10 weeks) with a single 320 mg intramuscular dose of the drug.[5] The tmax (time to maximal concentrations) for estradiol was about 16 days.[5]
In light of the fact that phosphatases, which cleave PEP into estradiol and phosphoric acid, are present in most tissues in the body, it has been said that the long terminal half-life and slow release of PEP are somewhat surprising.[31] Research has found that PEP acts as an inhibitor of alkaline phosphatase in vitro, and it is thought that PEP may inhibit its own metabolism.[31]
Chemistry
PEP is a synthetic estrane steroid and the C17β phosphoric acid (phosphate) ester of estradiol (estradiol 17β-phosphate) in the form of a polymer.[8][9][31][38] It is also known as estradiol polymer with phosphoric acid or as estradiol 17β-phosphate polymer, as well as estra-1,3,5(10)-triene-3,17β-diol 17β-phosphate polymer.[8][9][31][38] It has been determined via ultracentrifugation that the mean molecular weight of PEP corresponds to a chain length of approximately 13 repeat units of estradiol 17β-phosphate.[31] A substance closely related to PEP is polytestosterone phloretin phosphate, a testosterone ester in the form of a polymer.[39]
Solubility
PEP is of very low solubility in water, acetone, chloroform, dioxane, and ethanol, but dissolves readily in bases, especially in aqueous pyridine.[34]
Synthesis


Like polyphosphates of polyphenols, PEP can be prepared from the monomer (in this case estradiol) and phosphoryl chloride. The latter reacts with both the phenolic hydroxyl group in position 3 and the aliphatic one in position 17β. The molecular mass of the resulting polymer can be controlled by interrupting the reaction after a given time: the longer the reaction is allowed to continue, the higher the mass.[37][40]
History
Pharmacological experiments on estradiol phosphates conducted around 1950 gave rise to the hypothesis that estradiol 3,17β-diphosphate acted as an inhibitor of kidney alkaline phosphatase.[37] When the same scientists wanted to synthesize simple phosphates of phloretin, a compound found in apple tree leaves,[41] they accidentally created a polymer instead.[40] This was later shown to exhibit the same anti-phosphatase properties as estradiol diphosphate, and so it was hypothesized that the original finding was due to contamination with estradiol phosphate polymers.[37] Consequently, these polymers were studied in more detail, which resulted in the development of PEP as early as 1953[10] and its subsequent introduction for medical use in 1957 in the United States.[11][12]
Society and culture
Generic names
Polyestradiol phosphate is the generic name of the drug and its INN and BAN.[8][9][27] It is also known by its developmental code name Leo-114.[8][27]
Brand names
PEP is marketed exclusively under the brand name Estradurin or Estradurine.[8][27]
Availability

PEP has been marketed in the United States and widely throughout Europe, including in Austria, Belgium, the Czech Republic, Denmark, Finland, Germany, Italy, the Netherlands, Norway, Russia, Spain, Sweden, Switzerland, Ukraine, and the United Kingdom.[8][18][1][42][43][18][12] It is no longer available in the United States and possibly certain other countries however,[11][13] but is still known to be marketed in Austria, Belgium, Denmark, Finland, the Netherlands, Norway, Sweden, and Switzerland.[27][42][43][1]
Research
PEP has been studied as a means of hormonal breast enhancement in women.[44]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 http://pharmanovia.com/product/estradurin/
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. PMID 10230677.
- 1 2 3 4 5 6 7 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- 1 2 3 4 5 6 7 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057.
- ↑ Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ↑ Mikkola, A; Aro, J; Rannikko, S; Ruutu, M; Finnprostate, Group (March 2007). "Ten-year survival and cardiovascular mortality in patients with advanced prostate cancer primarily treated by intramuscular polyestradiol phosphate or orchiectomy". Prostate. 67 (4): 447–55. doi:10.1002/pros.20547. PMID 17219379.
- 1 2 3 4 5 6 7 8 9 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 856–. ISBN 978-3-88763-075-1.
- 1 2 3 4 S. Gangolli (1999). The Dictionary of Substances and Their Effects: O-S. Royal Society of Chemistry. pp. 425–. ISBN 978-0-85404-833-5.
- 1 2 3 4 Steinbach T, Wurm FR (2015). "Poly(phosphoester)s: A New Platform for Degradable Polymers". Angew. Chem. Int. Ed. Engl. 54 (21): 6098–108. doi:10.1002/anie.201500147. PMID 25951459.
- 1 2 3 4 "Drugs@FDA: FDA Approved Drug Products: Estradurin". United States Food and Drug Administration. Retrieved 24 June 2018.
- 1 2 3 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 2934–2935. ISBN 978-0-8155-1856-3.
- 1 2 Mosby (11 February 2009). Mosby's Pocket Dictionary of Medicine, Nursing & Health Professions. Elsevier Health Sciences. pp. 3672–. ISBN 0-323-06604-6.
- 1 2 3 W, Jasek, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 2992–4. ISBN 978-3-85200-181-4.
- 1 2 3 4 Sayed Y, Taxel P (2003). "The use of estrogen therapy in men". Curr Opin Pharmacol. 3 (6): 650–4. doi:10.1016/j.coph.2003.07.004. PMID 14644018.
- 1 2 3 4 Hedlund PO, Henriksson P (2000). "Parenteral estrogen versus total androgen ablation in the treatment of advanced prostate carcinoma: effects on overall survival and cardiovascular mortality. The Scandinavian Prostatic Cancer Group (SPCG)-5 Trial Study". Urology. 55 (3): 328–33. doi:10.1016/s0090-4295(99)00580-4. PMID 10699602.
- ↑ Deepinder F, Braunstein GD (2012). "Drug-induced gynecomastia: an evidence-based review". Expert Opinion on Drug Safety. 11 (5): 779–95. doi:10.1517/14740338.2012.712109. PMID 22862307.
Treatment with estrogen has the highest incidence of gynecomastia, at 40 – 80%, anti-androgens, including flutamide, bicalutamide and nilutamide, are next, with a 40 – 70% incidence, followed by GnRH analogs (goserelin, leuprorelin) and combined androgen deprivation, both with incidences of 13% each.
- 1 2 3 Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 455–. ISBN 978-3-7692-2114-5.
- ↑ Burkhard Helpap; Herbert Rübben (12 March 2013). Prostatakarzinom — Pathologie, Praxis und Klinik: Pathologie, Praxis und Klinik. Springer-Verlag. pp. 126–. ISBN 978-3-642-72110-6.
- 1 2 3 4 5 6 7 8 9 10 11 Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer—a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- 1 2 3 4 5 6 7 8 Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384.
- 1 2 3 4 Waun Ki Hong; James F. Holland (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 753–. ISBN 978-1-60795-014-1.
- ↑ Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- ↑ Langley RE, Cafferty FH, Alhasso AA, Rosen SD, Sundaram SK, Freeman SC, Pollock P, Jinks RC, Godsland IF, Kockelbergh R, Clarke NW, Kynaston HG, Parmar MK, Abel PD (April 2013). "Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09)". Lancet Oncol. 14 (4): 306–16. doi:10.1016/S1470-2045(13)70025-1. PMC 3620898. PMID 23465742.
- ↑ Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacol. Sin. 22 (2): 148–54. PMID 11741520.
- 1 2 3 4 5 https://www.drugs.com/international/polyestradiol-phosphate.html
- ↑ Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ↑ Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Lehrbuch der Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 508–. ISBN 978-3-662-00526-2.
- ↑ Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- 1 2 3 4 5 6 7 8 Gunnarsson PO, Norlén BJ (1988). "Clinical pharmacology of polyestradiol phosphate". Prostate. 13 (4): 299–304. doi:10.1002/pros.2990130405. PMID 3217277.
- 1 2 3 4 5 6 7 von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function—metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738.
- ↑ Gokhan Ozyigit; Ugur Selek (1 August 2017). Principles and Practice of Urooncology: Radiotherapy, Surgery and Systemic Therapy. Springer. pp. 334–. ISBN 978-3-319-56114-1.
The castrate level was defined as testosterone being less than 50 ng/dL (1.7 nmol/L), many years ago. However contemporary laboratory testing methods showed that the mean value after surgical castration is 15 ng/dL [1]. Thus, recently the level is defined as being less than 20 ng/dL (1 nmol/L).
- 1 2 3 4 Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-98-46-3.
- ↑ Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R, von Schoultz B (1989). "A comparison of androgen status in patients with prostatic cancer treated with oral and/or parenteral estrogens or by orchidectomy". Prostate. 14 (2): 177–82. doi:10.1002/pros.2990140210. PMID 2523531.
- ↑ Henriksson P, Carlström K, Pousette A, Gunnarsson PO, Johansson CJ, Eriksson B, Altersgård-Brorsson AK, Nordle O, Stege R (1999). "Time for revival of estrogens in the treatment of advanced prostatic carcinoma? Pharmacokinetics, and endocrine and clinical effects, of a parenteral estrogen regimen". Prostate. 40 (2): 76–82. doi:10.1002/(sici)1097-0045(19990701)40:2<76::aid-pros2>3.0.co;2-q. PMID 10386467.
- 1 2 3 4 Diczfalusy, E (April 1954). "Poly-estradiol phosphate (PEP); a long-acting water soluble estrogen". Endocrinology. 54 (4): 471–7. doi:10.1210/endo-54-4-471. PMID 13151143.
- 1 2 Johansson, CJ; Gunnarsson, PO (June 2000). "Pharmacodynamic model of testosterone suppression after intramuscular depot estrogen therapy in prostate cancer". Prostate. 44 (1): 26–30. doi:10.1002/1097-0045(20000615)44:1<26::AID-PROS4>3.0.CO;2-P. PMID 10861754.
- ↑ US patent 2928849, Hogberg Knut Bertil; Ferno Ove Birger & Linderot Torsten Ove Enok et al., "High-molecular weight derivatives of steroids containing hydroxyl groups and method of producing the same", published 15 March 1960, assigned to Leo Ab
- 1 2 Diczfalusy, E; Fernö, O; Fex, H; Högberg, B; Linderot, T; Rosenberg, Th (1953). "Synthetic high molecular weight enzyme inhibitors. I. Polymeric phosphates of phloretin and related compounds" (PDF). Acta Chem Scand. 7 (6): 921–7. doi:10.3891/acta.chem.scand.07-0913.
- ↑ Picinelli, A; Dapena, E; Mangas, JJ (1995). "Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study". Journal of Agricultural and Food Chemistry. 43 (8): 2273–78. doi:10.1021/jf00056a057.
- 1 2 http://www.micromedexsolutions.com
- 1 2 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- ↑ Hartmann BW, Laml T, Kirchengast S, Albrecht AE, Huber JC (1998). "Hormonal breast augmentation: prognostic relevance of insulin-like growth factor-I". Gynecol. Endocrinol. 12 (2): 123–7. doi:10.3109/09513599809024960. PMID 9610425.
Further reading
- Gunnarsson PO, Norlén BJ (1988). "Clinical pharmacology of polyestradiol phosphate". Prostate. 13 (4): 299–304. doi:10.1002/pros.2990130405. PMID 3217277.
- Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. PMID 10230677.