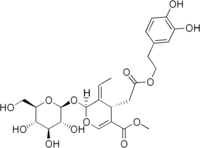
Oleuropein
 | |
| Names | |
|---|---|
| IUPAC name
(4S,5E,6S)-4-{2-[2-(3,4-dihydroxyphenyl)ethoxy]-2-oxoethyl}-5-ethylidene-6-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)-2-tetrahydropyranyl]oxy}-4H-pyran-3-carboxylic acid methyl ester | |
| Other names
2-(3,4-Dihydroxyphenyl)ethyl [(2S,3E,4S)-3-ethylidene-2-(β-D-glucopyranosyloxy)-5-(methoxycarbonyl)-3,4-dihydro-2H-pyran-4-yl]acetate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.046.466 |
PubChem CID |
|
| |
| |
| Properties | |
| C25H32O13 | |
| Molar mass | 540.51 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Oleuropein is a glycosylated seco-iridoid, a type of phenolic bitter compound found in green olives, olive leaves, and argan oil.[1][2][3] During processing of inedible green olives for human consumption as table olives, oleuropein is removed from olives by immersion in lye.[4]
Basic research
In preliminary laboratory research, oleuropein had activity as an agonist of the G-protein estrogen receptor.[5] Other basic research is examining whether oleuropein and other olive polyphenols have pharmacological properties and potential anti-disease activity,[6] none of which is proved to exist in humans as of 2017.[2]
See also
References
- ↑ Bendini, A; Cerretani, L; Carrasco-Pancorbo, A; Gómez-Caravaca, A. M.; Segura-Carretero, A; Fernández-Gutiérrez, A; Lercker, G (2007). "Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade". Molecules (Basel, Switzerland). 12 (8): 1679–719. doi:10.3390/12081679. PMID 17960082.
- 1 2 Rigacci, S; Stefani, M (2016). "Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans". International Journal of Molecular Sciences. 17 (6): 843. doi:10.3390/ijms17060843. PMC 4926377. PMID 27258251.
- ↑ Z. Charrouf and D. Guillaume (2007). "Phenols and Polyphenols from Argania spinosa". American Journal of Food Technology. 2 (7): 679–683. doi:10.3923/ajft.2007.679.683.
- ↑ "How olives are made". California Olive Committee. 2017. Retrieved 5 August 2017.
- ↑ Prossnitz, Eric R.; Barton, Matthias (2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Molecular and Cellular Endocrinology. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. ISSN 0303-7207. PMC 4040308. PMID 24530924.
- ↑ Taamalli, A; Arráez-Román, D; Zarrouk, M; Valverde, J; Segura-Carretero, A; Fernández-Gutiérrez, A (2012). "The occurrence and bioactivity of polyphenols in Tunisian olive products and by-products: A review". Journal of Food Science. 77 (4): R83–92. doi:10.1111/j.1750-3841.2011.02599.x. PMID 22352878.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.