COVID-19 testing
COVID-19 testing involves analyzing samples to assess the current or past presence of SARS-CoV-2. The two main branches detect either the presence of the virus or of antibodies produced in response to infection.[1][2] Tests for viral presence are used to diagnose individual cases and to allow public health authorities to trace and contain outbreaks. Antibody tests instead show whether someone once had the disease. They are not useful for diagnosing current infections because antibodies may not develop for weeks after infection.[3] It is used to assess disease prevalence, which aids the estimation of the infection fatality rate.[4]
| Part of a series on the |
| COVID-19 pandemic |
|---|
 |
|
|
|
International response |
|
Medical response |
|
|
|

Individual jurisdictions have adopted varied testing protocols, including whom to test, how often to test, analysis protocols, sample collection and the uses of test results.[5][6][7] This variation has likely significantly impacted reported statistics, including case and test numbers, case fatality rates and case demographics.[8][9][10]
Test analysis is often performed in automated, high-throughput, medical laboratories by medical laboratory scientists. Alternatively, point-of-care testing can be done in physician's offices, workplaces, institutional settings or transit hubs.
Methods

Positive viral tests indicate a current infection, while positive antibody tests indicate a prior infection.[12] Other techniques include a CT scan, checking for elevated body temperature or checking for low blood oxygen level.
Virus
Reverse transcription polymerase chain reaction
Polymerase chain reaction (PCR) is a process that amplifies (replicates) a small, well-defined segment of DNA many hundreds of thousands of times, creating enough of it for analysis. Test samples are treated with certain chemicals[13][14] that allow DNA to be extracted. Reverse transcription converts RNA into DNA.
Reverse transcription polymerase chain reaction (RT-PCR) first uses reverse transcription to obtain DNA, followed by PCR to amplify that DNA, creating enough to be analyzed.[14] RT-PCR can thereby detect SARS-CoV-2, which contains only RNA. The RT-PCR process generally requires a few hours.[15]
Real-time PCR (qPCR)[16] provides advantages including automation, higher-throughput and more reliable instrumentation. It has become the preferred method.[17][18]
The combined technique has been described as real-time RT-PCR[19] or quantitative RT-PCR[20] and is sometimes abbreviated qRT-PCR[21] rRT-PCR[22] or RT-qPCR,[23] although sometimes RT-PCR or PCR are used. The Minimum Information for Publication of Quantitative Real-Time PCR Experiments (MIQE) guidelines propose the term RT-qPCR,[24] but not all authors adhere to this.
Samples can obtained by various methods, including a nasopharyngeal swab, sputum (coughed up material),[25] throat swabs,[26] deep airway material collected via suction catheter[26] or saliva.[27][28] Drosten et al. remarked that for 2003 SARS, "from a diagnostic point of view, it is important to note that nasal and throat swabs seem less suitable for diagnosis, since these materials contain considerably less viral RNA than sputum, and the virus may escape detection if only these materials are tested."[29]
The likelihood of detecting the virus depends on collection method and how much time has passed since infection. According to Drosten tests performed with throat swabs are reliable only in the first week. Thereafter the virus may abandon the throat and multiply in the lungs. In the second week, sputum or deep airways collection is preferred.[26]
Collecting saliva may be as effective as nasal and throat swabs,[27] although this is not certain.[30][28] Sampling saliva may reduce the risk for health care professionals by eliminating close physical interaction.[31] It is also more comfortable for the patient.[32] Quarantined people can collect their own samples.[31] A saliva test's diagnostic value depends on sample site (deep throat, oral cavity, or salivary glands).[28] One study found that saliva yielded greater sensitivity and consistency when compared with swab samples.[33]
Viral burden measured in upper respiratory specimens declines after symptom onset.[34]
.jpg) Demonstration of a nasopharyngeal swab for COVID-19 testing
Demonstration of a nasopharyngeal swab for COVID-19 testing.jpg) Demonstration of a throat swab for COVID-19 testing
Demonstration of a throat swab for COVID-19 testing- A PCR machine
Isothermal amplification assays
Isothermal nucleic acid amplification tests also amplify the virus's genome. They are faster than PCR because they don't involve repeated heating and cooling cycles. These tests typically detect DNA using fluorescent tags, which are read out with specialized machines. CRISPR gene editing technology was modified to perform the detection: if the CRISPR enzyme attaches to the sequence, it colors a paper strip. The researchers expect the resulting test to be cheap and easy to use in point-of-care settings.[35][36] The test amplifies RNA directly, without the RNA-to-DNA conversion step of RT-PCR.[37]
Antigen
An antigen is the part of a pathogen that elicits an immune response. Antigen tests look for antigen proteins from the viral surface. In the case of a coronavirus, these are usually proteins from the surface spikes.[38] One of the challenges is to find a target unique to SARS-CoV-2.[39]
Antigen tests may be one way to scale up testing to much greater levels.[38] Isothermal nucleic acid amplification tests can process only one sample at a time per machine. RT-PCR tests are accurate but require too much time, energy and trained personnel to run the tests.[38] "There will never be the ability on a [PCR] test to do 300 million tests a day or to test everybody before they go to work or to school," Deborah Birx, head of the White House Coronavirus Task Force, said on 17 April 2020. "But there might be with the antigen test."[40]
A nasopharyngeal swab is exposed to paper strips containing artificial antibodies designed to bind to coronavirus antigens. Antigens bind to the strips and give a visual readout. The process takes less than 30 minutes, can deliver results at point of care, and does not require expensive equipment or extensive training.[38]
Swabs of respiratory viruses often lack enough antigen material to be detectable.[39] This is especially true for asymptomatic patients who have little if any nasal discharge. Viral proteins are not amplified in an antigen test.[38][41] According to the WHO the sensitivity of similar antigen tests for respiratory diseases like the flu ranges between 34% and 80%. "Based on this information, half or more of COVID-19 infected patients might be missed by such tests, depending on the group of patients tested," the WHO said. Many scientists doubt whether an antigen test can be made reliable enough in time to be useful against COVID-19.[41] According to the FDA, positive results from antigen tests are highly accurate, but there is a higher chance of false negatives, so negative results do not rule out infection. Therefore, negative results from an antigen test may need to be confirmed with a PCR test.[42]
Imaging
Typical visible features on CT initially include bilateral multilobar ground-glass opacities with a peripheral or posterior distribution.[43] Subpleural dominance, crazy paving, and consolidation may develop as the disease evolves.[43][44] Chest CT scans and chest x-rays are not recommended for diagnosing COVID-19. Radiologic findings in COVID-19 lack specificity.[45][43]
Serology
Serology tests test blood and can identify once-infected people who have recovered.[46]
Part of the immune system's response to infection is the production of antibodies, including IgM and IgG. According to the FDA, IgM antibodies to SARS-CoV-2 are generally detectable several days after initial infection, although levels over the course of infection and beyond are not well characterized.[47] SARS-CoV-2 IgG antibodies generally become detectable 10–14 days after infection, sometimes earlier, and normally peak around 28 days after infection onset.[48][49] Antibodies emerge too late to serve as acute infection markers. Antibodies for some diseases persist in the bloodstream for many years, while others quickly fade away.[38]
Antibody tests can be used to estimate the once-infected percentage of a population. SARS-CoV-2 antibodies' potency and protective period are unknown.[4][50]
Types
Rapid diagnostic test (RDT)
RDTs typically use a small, portable, positive/negative lateral flow assay that can be executed at point of care. RDTs may process blood samples, saliva samples, or nasal swab fluids. RDTs produce colored lines to indicate positive or negative results.[51]
Enzyme-linked immunosorbent assay (ELISA)
ELISAs can be qualitative or quantitative and generally require a lab. These tests usually use whole blood, plasma, or serum samples. A plate is coated with a viral protein, such as a SARS-CoV-2 spike protein. Samples are incubated with the protein, allowing any antibodies to bind to it. The antibody-protein complex can then be detected with another wash of antibodies that produce a color/fluorescent readout.[51]
Neutralization assay
Neutralization assays assess whether sample antibodies prevent viral infection in test cells. These tests sample blood, plasma or serum. The test cultures cells that allow viral reproduction (e.g., VeroE6 cells). By varying antibody concentrations, researchers can visualize and quantify how many test antibodies block virus replication.[51]
Chemiluminescent immunoassay
Chemiluminescent immunoassays are quantitative lab tests. They sample blood, plasma, or serum. Samples are mixed with a known viral protein, buffer reagents and specific, enzyme-labeled antibodies. The result is luminescent. A chemiluminescent microparticle immunoassay uses magnetic, protein-coated microparticles. Antibodies react to the viral protein, forming a complex. Secondary enzyme-labeled antibodies are added and bind to these complexes. The resulting chemical reaction produces light. The radiance is used to calculate the number of antibodies. This test can identify multiple types of antibodies, including IgG, IgM, and IgA.[51]
Neutralizing vs binding antibodies
Most if not all large scale COVID-19 antibody testing looks for binding antibodies only and does not measure the more important neutralizing antibodies (NAb).[52][53][54] A NAb is an antibody that defends a cell from an infectious particle by neutralizing its biological effects. Neutralization renders the particle no longer infectious or pathogenic.[55] A binding antibody binds to the pathogen but the pathogen remains infective; the purpose can be to flag the pathogen for destruction by the immune system.[56] It may even enhance infectivity by interacting with receptors on macrophages.[57] Since most COVID-19 antibody tests return a positive result if they find only binding antibodies, these tests cannot indicate that the subject has generated protective NAbs that protect against re-infection.[53][54]
It is expected that binding antibodies imply the presence of NAbs[54] and for many viral diseases total antibody responses correlate somewhat with NAb responses[58] but this is not established for COVID-19. A study of 175 recovered patients in China who experienced mild symptoms reported that 10 individuals had no detectable NAbs at discharge, or thereafter. How these patients recovered without the help of NAbs and whether they were at risk of re-infection was not addressed.[53] An additional source of uncertainty is that even if NAbs are present, viruses such as HIV can evade NAb responses.[52]
Studies have indicated that NAbs to the original SARS virus (the predecessor to the current SARS-CoV-2) can remain active for two years[59] and are gone after six years.[60] Nevertheless, memory cells including Memory B cells and Memory T cells[61] can last much longer and may have the ability to reduce reinfection severity.[60]
.jpg)
.jpg) Blood from pipette is then placed onto a COVID-19 rapid diagnostic test device.
Blood from pipette is then placed onto a COVID-19 rapid diagnostic test device.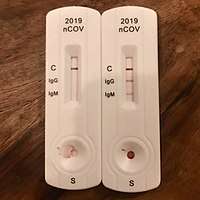
Other tests
Following recovery, many patients no longer have detectable viral RNA in upper respiratory specimens. Among those who do, RNA concentrations three days following recovery are generally below the range in which replication-competent virus has been reliably isolated.[62]
No clear correlation has been described between length of illness and duration of post-recovery shedding of viral RNA in upper respiratory specimens.[63]
Infectivity
Infectivity is indicated by the basic reproduction number (R0, pronounced "R naught") of the disease.[64] SARS-CoV-2 is estimated to have an R0 of 2.2 to 2.5.[65][66] This means that in a population where all individuals are susceptible to infection, each infected person is expected to infect 2.2 to 2.5 others in the absence of interventions.[67] R0 can vary according factors such as geography, population demographics and density.[68] In New York state R0 was estimated to be 3.4 to 3.8.[69]
On average, an infected person begins showing symptoms five days after infection (the "incubation period") and can infect others beginning two to three days before that.[65][70] One study reported that 44% of viral transmissions occur within this period.[65][71] According to CDC, a significant number of infected people who never show symptoms are nevertheless contagious.[71][66] In vitro studies have not found replication-competent virus after 9 days from infection.[72] The statistically estimated likelihood of recovering replication-competent virus approaches zero by 10 days.[73]
Infectious virus has not been cultured from urine or reliably cultured from feces;[74] these potential sources pose minimal if any risk of transmitting infection and any risk can be sufficiently mitigated by good hand hygiene.
Patterns and duration of illness and infectivity have not been fully described. However, available data indicate that SARS-CoV-2 RNA shedding in upper respiratory specimens declines after symptom onset. At 10 days recovery of replication-competent virus in viral culture (as a proxy of the presence of infectious virus) approaches zero. Although patients may produce PCR-positive specimens for up to six weeks,[75] it remains unknown whether these samples hold infectious virus. After clinical recovery, many patients do not continue to shed. Among recovered patients with detectable RNA in upper respiratory specimens, concentrations after three days are generally below levels where virus has been reliably cultured. These data were generated from adults across a variety of age groups and with varying severity of illness. Data from children and infants were not available.[72]
History
January
Scientists from China first released information on the viral genome on 11 January 2020. That day the Malaysian Institute for Medical Research (IMR) produced "primers and probes" specific to a SARS-CoV-2 RT-PCR test.[77] The IMR's materials were used to diagnose Malaysia's first patient on 24 January.[78] BGI Group was one of the first companies to receive emergency use approval from China's National Medical Products Administration for a nucleic acid test.[79]
Public Health England announced a test on the 10th,[80] using a real-time RT-PCR (RdRp gene) assay based on oral swabs.[81] The test detected the presence of any type of coronavirus, including specifically identifying SARS-CoV-2. It was rolled out to twelve laboratories across the United Kingdom on 10 February.[82]
The first case in South Korea was confirmed on 19 January.[83]
The German nucleic acid testing protocol was published on the 17th. Another early PCR test was developed by Charité University hospital in Berlin, working with academic collaborators in Europe and Hong Kong, and published on the 23rd. It used rtRT-PCR, and formed the basis of 250,000 kits distributed by the World Health Organization (WHO).[84]
In the US, the CDC developed its SARS-CoV-2 Real Time PCR Diagnostic Panel.[85] The protocol became available on the 28th.[86] One of three tests in early kits failed due to faulty reagents.
In Russia, the first COVID‑19 test was developed by the State Research Center of Virology and Biotechnology VECTOR. Production began on 24 January.[87]
February
CDC refused to let other labs process tests that month; allowing an average of fewer than 100 samples/day to be processed. Tests using two components were not determined to be reliable until the 28th, and only then were state and local laboratories permitted to begin testing.[88] The test was approved by the FDA under an EUA.South Korean company Kogenebiotech's clinical grade, nucleic acid test (PowerChek Coronavirus) was approved by Korea Centers for Disease Control and Prevention (KCDC) on 4 February.[89]
In Wuhan BGI opened a makeshift 2000-sq-meter emergency detection laboratory named "Huo-Yan" (Chinese: 火眼, "Fire Eye") on the 5th.[90][91] It processed more than 10,000 samples/day.[92][91] Construction required 5 days.[93] The Wuhan Laboratory was followed by Huo-Yan labs in Shenzhen, Tianjin, Beijing, and Shanghai, in a total of 12 cities across China.
On 11 February the test was approved by the Federal Service for Surveillance in Healthcare.[94]
March
By 4 March China reached 50,000 tests per day.[95]
On the 16th WHO called for ramping up testing programmes as the best way to slow the spread.[96][97]
Shortages of reagent and other supplies became a bottleneck for mass testing in the EU and UK[98] and the US.[99][100]
On 12 March, Mayo Clinic announced a nucleic acid test.[101]
Early in March, China reported accuracy problems with its PCR tests.[102] A study examined 1070 samples from 205 Wuhan patients and reported varied sensitivity according to the methods and location of sample collection. Samples from bronchoalveolar lavage fluid specimens returned the highest sensitivity.[103] The authors argued that CT scans showed even higher sensitivity.[104]
US commercial labs began testing in early March. As of the 5th LabCorp announced nationwide availability of COVID‑19 testing based on RT-PCR.[105] Quest Diagnostics made nationwide testing available as of 9 March.[106] US testing demand grew rapidly, causing backlogs of hundreds of thousands of tests at private US labs. Supplies of swabs and chemical reagents continued strained.[107] On 25 May, the US required each state to take responsibility for meeting its testing needs.[108] In March, the FDA issued EUAs for nucleic acid tests to Hologic (3/16),[109] Abbott Laboratories (3/18),[110] Thermo Fisher Scientific (3/19)[111] Cepheid (3/21)[112][113] and LabCorp (4/30).[110]
On 27 March the FDA issued an EUA for a test using Abbott Laboratories' ID Now assay.[114]
By 31 March United Arab Emirates was testing more of its population per head than any other country.[115] UAE implemented a combination of drive-through sample collection, and a mass-throughput laboratory from Group 42 and BGI. The lab conduced tens of thousands RT-PCR tests per day and was the first to be operational at that scale other than China.[116]
Several European countries initially conducted more tests than the US.[117][118] By 19 March drive-in tests were offered in several large cities.[119] As of 26 March, German Health Minister Jens Spahn estimated that Germany was conducting 200,000 tests per week.[120] As of the end of March at least 483,295 samples were tested and 33,491 (6.9%) had tested positive.[121]
80% of test kits that Czechia purchased from China gave wrong results.[122][123] Slovakia purchased 1.2 million antibody-based test kits from China that were found to be inaccurate.[124] China accused Czechia and Slovakia of incorrect use of those tests.[125] Ateş Kara of the Turkish Health Ministry said the test kits Turkey purchased from China had a "high error rate".[126][127]
Germany has a large medical diagnostics industry, with more than a hundred testing labs that provided the technology and infrastructure to enable rapid increases in testing. Costs are borne by insurance when the test is ordered by a physician.[128] According to the president of the Robert Koch Institute, as of 22 March, Germany had capacity for 160,000 tests per week.[129]
Spain purchased test kits from Chinese firm Shenzhen Bioeasy Biotechnology Co Ltd, but found that results were unacceptable. The maker explained that the incorrect results may stem from failure to collect samples or use the kits correctly. The Spanish ministry switched to another vendor, Shenzhen Bioeasy.[130]
By month end, testing had surpassed 200k/week.[131]
April
The FDA gave an EUA for the US' first antibody test on the 2nd.[132][50]
As of 7 April, WHO had accepted two diagnostic tests for procurement under the Emergency Use Listing procedure (EUL).[133]
On 13 April, Health Canada approved a nucleic acid test from Spartan Bioscience. Institutions may "test patients" with a handheld DNA analyzer "and receive results without having to send samples away to a [central] lab".[134][135]
By the start of April, the United Kingdom was delivering around 10,000 swab tests per day.[136] The British NHS announced that it was piloting a scheme to test suspected cases at home, to remove the risk of one patient infecting others at a hospital or disinfecting an ambulance used to transport a patient.[137]
The UK purchased 3.5 million antibody test kits from China, but in early April 2020 announced these were not usable.[138][139] On 21 April 2020, the Indian Council of Medical Research (ICMR) advised Indian states to stop using test kits purchased from China after receiving complaints from one state. Rajasthan health minister Raghu Sharma on 21 April said the kits gave only 5.4 percent accurate results.[140]
Antibody survey results found from 2% to 30% positive.[141] On preliminary data, WHO concluded that 2% to 3% of the world population had developed antibodies.[142]
By month end, testing had surpassed 750k/week.[131]
May
In May antibody tests were conducted on 5,603 major league baseball employees and 0.7% tested positive, showing that they had been infected. 70% of those who tested positive had had no symptoms.[143][144][145] The US was conducting an average of 2.5 million tests per week for the week ending 17 May. This grew to 3.2 million by 14 June.[146][147]
Attempts to culture virus from upper respiratory specimens were largely unsuccessful when viral burden is low but detectable (i.e., Ct values higher than 33-35).[72]
On 3 May, Roche received an EUA for a selective ELISA serology test.[148][149]
On 8 May, the FDA granted its first EUA for antigen test: "Sofia 2 SARS Antigen FIA" by Quidel Corp.[150][42]
The FDA announced on 14 May a review of 15 adverse event reports about the Abbott ID Now device for low sensitivity.[151]
On 21 May, researchers at Ben-Gurion University in Israel reported a one-minute coronavirus test with 90% accuracy, based on the "change in the resonance in the THz spectral range" shown by the coronavirus through THz spectroscopy.[152]
Nearly two million antibody tests imported into Australia and costing $20 million were declared unusable.[153][154][155]
In early May Harvard's Global Health Institute estimated that the US needed to test more than 900k per day.[156][157] Other recommendations ranged up to 23m per day.[158][159][160][161]
On 29 May Siemens received an EUA for its anti-spike RBD-targeting serology test that it believes detects neutralizing antibodies.[162]
By month end, testing had surpassed 1.4m/week.[131]
June
In early June, researchers announced a nucleic acid diagnostic test using reverse transcription-loop-mediated isothermal amplification (RT-LAMP), an existing technology used in pathogenic microorganism identification, genetically modified ingredients, tumor detection, and embryo sex identification. The test identified virus in samples of serum, urine, saliva, oropharyngeal swabs and nasopharyngeal swabs. Once commercialized the test has the potential to provide rapid (30-45 minute) diagnosis at point of care. The test was 100% selective and highly sensitive, detecting virus at a concentration of .06 fg/ml.[163]
As of 14 June 2020, the percentage testing positive in the US as a whole had fallen below 5%.[164] As of late June, test numbers crossed 600k/day.[146]
Testing protocols

Drive-through testing
In drive-through testing, a healthcare professional takes a sample using appropriate precautions, such as wearing PPE.[165][166] Drive-through centers helped South Korea accelerate its testing program.[167]
Home collection
In Hong Kong test subjects can stay home and receive a specimen tube. They spit into it, return it and later get the result.[168]
Pooled testing
In Israel, researchers at Technion and Rambam Hospital developed a method for testing samples from 64 patients simultaneously, by pooling the samples and only testing further if the combined sample was positive.[169][170][171] Pool testing was then adopted in Israel, Germany, South Korea,[172] Nebraska,[173] China[174] and the Indian states of Uttar Pradesh,[175] West Bengal,[176] Punjab,[177] Chhattisgarh[178] and Maharashtra.[179]
Open source, multiplexed designs released by Origami Assays can test as many as 1122 patient samples using only 93 assays.[180] These balanced designs can be run in small laboratories without robotic liquid handlers.
Multi-tiered testing
One study proposed a rapid immune response assay as a screening test, with a confirmatory nucleic acid test for diagnosis, followed by a rapid antibody test to determine course of action and assess population exposure/herd immunity.[181]
Required volume
Required testing levels are a function of disease spread. The more the cases, the more tests are needed to manage the outbreak. COVID-19 tends to grow exponentially at the beginning of an outbreak, meaning that the number of required tests initially also grows exponentially. If properly targeted testing grows more rapidly than cases, it can be contained.
WHO recommends increasing testing until fewer than 10% are positive in any given jurisdiction.[182]
United States
Economist Paul Romer reported that the US has the technical capacity to scale up to 20 million tests per day, which is his estimate of the scale needed to fully remobilize the economy.[159] The Edmond J. Safra Center for Ethics estimated on 4 April that this capacity could be available by late July.[183] Romer pointed to single-molecule real-time sequencing equipment from Pacific Biosciences[184][159] and to the Ion Torrent Next-Generation Sequencing equipment from ThermoFisher Scientific.[185][159] According to Romer, "Recent research papers suggest that any one of these has the potential to scale up to millions of tests per day." This plan requires removing regulatory hurdles. Romer estimated that $100 billion would cover the costs.[159]
Romer also claimed that high test accuracy is not required if tests are administered frequently enough. He ran model simulations in which 7% of the population is tested every day using a test with a 20% false negative rate and a 1% false positive rate. The average person would be tested roughly every two weeks. Those who tested positive would go into quarantine. Romer's simulation indicated that the fraction of the population that is infected at any given time (known as the attack rate) peaks reaches roughly 8% in about thirty days before gradually declining, in most runs reaching zero at 500 days, with cumulative prevalence remaining below 20%.[186]

Blue: CDC lab
Orange: Public health lab
Gray: Data incomplete due to reporting lag
Not shown: Testing at private labs; total exceeded 100,000 per day by 27 March.[187]
Available tests

Countries around the world developed tests independently and in partnership with others.
Nucleic acid tests
Tests developed in China, France, Germany, Hong Kong, Japan, the United Kingdom, and the US targeted different parts of the viral genome. WHO adopted the German system for manufacturing kits sent to low-income countries without the resources to develop their own.
PowerChek Coronavirus looks for the "E" gene shared by all beta coronaviruses, and the RdRp gene specific to SARS-CoV-2.[188]
.jpg)
Abbott Laboratories' ID Now nucleic acid test uses isothermal amplification technology.[114] The assay amplifies a unique region of the virus's RdRp gene; the resulting copies are then detected with "fluorescently-labeled molecular beacons".[189] The test kit uses the company's "toaster-size" ID Now device, which is widely deployed in the US.[190] The device can be used in laboratories or in point of care settings, and provides results in 13 minutes or less.[189]
Primerdesign offers its Genesig Real-Time PCR Coronavirus (COVID‑19). Cobas SARS-CoV-2 Qualitative assay runs on the Cobas® 6800/8800 Systems by Roche Molecular Systems. They are offered by the United Nations and other procurement agencies.
Antigen tests
Quidel's "Sofia 2 SARS Antigen FIA"[150][42] is a lateral flow test that uses monoclonal antibodies to detect the virus's nucleocapsid (N) protein.[191] The result is read out by the company's Sofia 2 device using immunofluorescence.[191] The test is simpler and cheaper but less accurate than nucleic acid tests. It can be deployed in laboratories or at point of care and gives results in 15 minutes.[150] A false negative result occurs if the sample's antigen level is positive but below the test's detection limit, requiring confirmation with a nucleic acid test.[191]
Serology (antibody) tests
Antibodies are usually detectable 14 days after the onset of the infection. Multiple jurisdictions survey their populations using these tests.[192][193] The test requires a blood draw.
Private US labs including Quest Diagnostics and LabCorp offer antibody testing upon request.[194]
Antibody tests are available in various European countries.[195]
Accuracy
Accuracy is measured in terms of specificity and selectivity. Test errors can be false positives (the test is positive, but the virus is not present) or false negatives, (the test is negative, but the virus is present).[197]
Sensitivity and specificity
Sensitivity indicates whether the test accurately identifies whether the virus is present. Each test requires a minimum level of viral load in order to produce a positive result. A 90% sensitive test will correctly identify 90% of infections, missing the other 10% (a false negative). Even relatively high sensitivity rates can produce high rates of false negatives in populations with low incidence rates.[197]
Specificity indicates how well-targeted the test is to the virus in question. Highly specific tests pick up only the virus in question. Non-selective tests pick up other viruses as well. A 90% specific test will correctly identify 90% of those who are uninfected, leaving 10% with a false positive result.[197]
Low-specificity tests have a low positive predictive value (PPV) when prevalence is low. For example, suppose incidence is 5%. Testing 100 people at random using a test that has a specificity of 95% would yield on average 5 people who are actually negative who would incorrectly test positive. Since 5% of the subjects actually are positive, another five would also test positive correctly, totaling 10 positive results. Thus, the PPV is 50%,[198] an outcome no different from a coin toss. In this situation retesting those with a positive result increases the PPV to 94.5%, meaning that only 4.5% of the second tests would return the incorrect result, on average less than 1 incorrect result.[199]
Causes of test error
Improper sample collection, exemplified by failure to acquire enough sample and failure to insert a swab deep into the nose. This results in insufficient viral load, one cause of low clinical sensitivity.
The time course of infection also affects accuracy. Samples may be collected before the virus has had a chance to establish itself or after the body has stopped its progress and begun to eliminate it.
Improper storage for too long a time can cause RNA breakdown and lead to wrong results as viral particles disintegrate.[200]
Improper design and manufacture can yield inaccurate results. Millions of tests made in China were rejected by various countries throughout the period of March 2020 through May 2020.
Test makers typically report the accuracy levels of their tests when seeking approval from authorities. In some jurisdictions, these results are cross-validated by additional assessments. Reported results may not be achieved in clinical settings due to such operational inconsistencies.
PCR-based test
.png)
RT-PCR is the most accurate diagnostic test.[102] It typically has high sensitivity and specificity in a laboratory setting: however, in one study sensitivity dropped to 66-88% clinically.[201]
In one study sensitivity was highest at week one (100%), followed by 89.3%, 66.1%, 32.1%, 5.4% and zero by week six.[202][203]
| Samples from ... | Positive rate |
|---|---|
| Bronchoalveolar lavage fluid specimens | 93% (n=14) |
| Sputum | 72% (n=75) |
| Nasal swabs | 63% (n=5) |
| Fibrobronchoscope brush biopsy | 46% (6/13) |
| Pharyngeal swabs | 32% (n=126) |
| Feces | 29% (n=44) |
| Blood | 1% (n=3) |
A Dutch CDC-led laboratory investigation compared 7 PCR kits.[204] Test kits made by BGI, R-Biopharm AG, BGI, KH Medical and Seegene showed high sensitivity.[205]
High sensitivity kits are recommended to assess people without symptoms, while lower sensitivity tests are adequate when diagnosing symptomatic patients.[204]
Confirmatory testing
The WHO recommends countries that do not have testing capacity and national laboratories with limited experience on COVID‑19 send their first five positives and the first ten negative COVID‑19 samples to one of the 16 WHO reference laboratories for confirmatory testing.[208][209] Out of the sixteen reference laboratories, seven are in Asia, five in Europe, two in Africa, one in North America and one in Australia.[210]
National responses
See also: International testing statistics US testing statistics
Iceland
Iceland[211] managed the pandemic with aggressive contact tracing, inbound travel restrictions, testing, and quarantining, but with less aggressive lock-downs.
Italy
Researchers tested the entire population of Vò, the site of Italy's first COVID‑19 death. They tested about 3,400 people twice, at an interval of ten days. About half the people testing positive had no symptoms. All discovered cases were quarantined. Along with restricting travel to the commune, new infections were completely eliminated.[212]
Japan
Unlike other Asian countries, Japan did not experience a pandemic of SARS or MERS, so the country's PCR testing system was not well developed.[213][214] Japan preferentially tested patients with severe illness and their close contacts at the beginning. Japan's Novel Coronavirus Expert Meeting chose cluster measures to identify infections clusters.[213][214] The Expert Meeting analyzed the outbreak from Wuhan and identified conditions leading to clusters (closed spaces, crowded spaces and close-contact), and asked people to avoid them.[214][215]
In January, contact tracers took action shortly after the first infection was found. Only administrative tests were carried out at first, until insurance began covering PCR tests on 6 March. Private companies began to test, and the test system gradually expanded.[213][216]
On 3 April, those with positive tests were legally permitted to recuperate at home or in a hotel if they had asymptomatic or mild illness, ending the hospital bed shortage.[217] The first wave (from China) was contained,[218] but a second wave (caused by returnees from Europe and the US) in mid-March led to spreading infection in April.[214] On 7 April, Japan declared a state of emergency, (less strict than a lockdown, because it did not block cities or restrict outings).[214][217][219] On 13 May, antigen test kits became covered by insurance, and were combined with a PCR test for diagnosis.[220][221]
Japan's PCR test count per capita remained far smaller than in some other countries even though its positive test rate was lower. Excess mortality was observed in March.[215][219][222] The Expert Meeting stated, "The Japanese health care system originally carries out pneumonia surveillance, allowing it to detect most of the severely ill patients who develop pneumonia. There are a large number of CT scanners in Japan and they have spread to small hospitals all over the country, so pneumonia patients are rarely missed. In that sense, it meets the same standards as other countries that mainly carry out PCR tests."[215][222] The group recommended using CT scans data and doctor's findings for diagnosis.[223][224] On the Diamond Princess cruise ship, many people who initially tested negative later tested positive. Half of coronavirus-positives there who remained mild or asymptomatic had pneumonia findings on CT scans and their CT image showed a frosted glass shadow that is characteristic of infection.[225][226]
Russia
On 27 April, Russia tested 3 million people and had 183,000 positive results.[227] On 28 April Anna Popova, head of Federal Service for Surveillance in Healthcare (Roszdravnadzor) stated that 506 laboratories were testing; that 45% of those who tested positive had no symptoms; that 5% of patients had a severe form; and 40% of infections were from family members. Illness improved from six days to one day after symptoms appeared. Antibody testing was carried out on 3,200 Moscow doctors, finding 20% immunity.[228]
Singapore
With contact tracing, inbound travel restrictions, testing, and quarantining, Singapore arrested the initial spread without complete lockdown.[229]
South Korea
South Korea's broad testing approach helped reduce spread. Testing capacity, largely in private sector labs, was built up over several years by the South Korean government in the early 2000s.[230]
The government exploited the resident registration number (RRN) system. Authorities mobilized young men who were eligible for military service as social service agents, security and public health doctors. Public health doctors were mainly dispatched to public health centers and life treatment centers where mildly ill patients were accommodated. They performed PCR tests and managed mild patients. Social service agents worked in pharmacies to fill staff shortages. Korea's 10k PCR tests per million residents was the world's highest as of 13 April rising to 20k by mid-June. Twenty-seven Korean companies exported test kits worth $48.6 million in March, and were asked to provide test kits or humanitarian assistance by more than 120 countries. Korean authorities set up a treatment center to isolate and manage patients with asymptomatic and minor illnesses in one facility in order to vacate hospital beds for the more severely ill.
Centers were sited mainly at national facilities and corporate training centers. The failure of Korea's MERS quarantine in May 2015 left Korea more prepared for COVID-19 than countries that did not face that pandemic. Then President Park Geun-hye allowed Korean CDC-approved private sector testing for infectious diseases in 2016. Korea already had a system for isolating, testing and treating infectious disease patients separately from others. Patients with respiratory illness but no epidemiological relevance were treated at the National Hospital, and those with epidemiological relevance were treated at selected clinics.[83][231][232][233][234][235][236][237][238]
Korea established a large scale drive-through/walk-through" test testing program. However, the most common method was “mobile examination”. In Daegu City, 54% of samples were collected by 23 March in home or hospital. Collecting samples door-to-door of avoided the risk of travel by possibly infected patients, but required additional staff. Korea solved the problem by drafting more than 2,700 public insurance doctors.[83][234][233]
The government disclosed personal information to the public via KCDC without patient consent. The authorities used digital surveillance to trace possible spread.[231][234][235][237][238][239][240][241][242] [243]
Taiwan
Health insurance IDs and national identification card numbers were used to trace contacts.[244][245][246][247]
United States
New York State
New York State's control measures consisted of PCR tests, stay-at-home measures and strengthening the healthcare system. On 29 February before its first case, the state allowed testing at the Wordsworth Center. They managed to convince the CDC to approve tests at state laboratories and the FDA to approve a test kit. As of 13 March the state was conducting more than 1,000 daily tests, growing to 10,000/day on 19 March. In April, the number exceeded 20,000. Many people queued at hospitals to get tested. On 21 March New York City health officials directed medical providers to test only those entering the hospital, for lack of PPE.[237][248][249][250][251]
USS Theodore Roosevelt
Following an outbreak, 94% of the 4,800 aircraft carrier crew were tested. Roughly 60 percent of the 600-plus sailors who tested positive were asymptomatic.[252] Five infected sailors who completed quarantine subsequently developed flu-like symptoms and again tested positive.[253]
Delayed testing
A shortage of trained medical laboratory scientists, assay reagents, analyzers, transport medium, and PPE coupled with high demand had limited initially limited the availability of testing and led to significantly increased turnaround times.
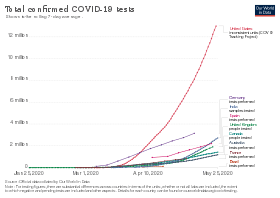
Statistics by country
Testing strategies vary by country and over time,[254] with some countries testing very widely,[7] while others have at times focused narrowly on only testing the seriously ill.[255] The country that tests only people showing symptoms will have a higher figure for "% (Confirmed cases as percentage of tested samples or tested cases)" than the country that also tests others.[256] If two countries are alike in every respect, including which people they test, the one that tests more people will have a higher "Confirmed / million people". Studies have also found that countries that test more, relative to the number of deaths, have lower estimated case fatality rates[8] and younger age distributions of cases.[10]
| Country | Date[lower-alpha 1] | Tested | Units[lower-alpha 2] | Confirmed (cases) |
% | Tested / million people |
Confirmed / million people |
Ref. |
|---|---|---|---|---|---|---|---|---|
| 31 May | 38,460 | samples | 15,205 | 39.5 | 988 | 391 | [257] | |
| 9 June | 16,716 | samples | 1,299 | 7.8 | 5,839 | 454 | [258] | |
| 20 June | 22,467 | samples | 11,631 | 51.8 | 515 | 267 | [259] | |
| 28 June | 329,036 | samples | 55,343 | 17.1 | 7,425 | 1,272 | [260] | |
| 24 May | 50,397 | 6,661 | 13.2 | 17,074 | 2,257 | [261] | ||
| 28 June | 2,379,175 | samples | 7,686 | 0.32 | 93,668 | 303 | [262] | |
| 29 June | 606,375 | 17,631 | 2.9 | 68,112 | 1,980 | [263] | ||
| 31 May | 298,648 | 5,494 | 1.8 | 30,173 | 555 | [264] | ||
| 21 June | 477,788 | 21,764 | 4.6 | 304,431 | 13,867 | [265] | ||
| 26 June | 699,941 | samples | 130,474 | 18.6 | 4,250 | 792 | [266] | |
| 23 May | 4,664 | 92 | 2.0 | 16,249 | 321 | [267] | ||
| 24 June | 901,904 | samples | 59,945 | 6.6 | 95,019 | 6,315 | [268] | |
| 23 May | 768,706 | samples | 56,810 | 7.4 | 66,752 | 4,933 | [269] | |
| 5 June | 18,821 | samples | 48 | 0.13 | 25,375 | 65 | [270] | |
| 20 June | 56,964 | cases | 23,512 | 41.3 | 4,984 | 2,057 | [271] | |
| 24 June | 86,273 | samples | 3,676 | 4.3 | 25,215 | 1,074 | [272] | |
| 26 June | 11,340,524 | samples | 1,280,054 | 11.3 | 53,965 | 6,091 | [273][274] | |
| 24 May | 74,096 | samples | 2,427 | 3.3 | 10,661 | 349 | [275] | |
| 21 May | 6,642 | samples | 814 | 12.3 | 318 | 39 | [276][277] | |
| 10 May | 10,268 | 2,579 | 25.1 | 387 | 97 | [278] | ||
| 26 June | 2,598,243 | cases | 102,794 | 4.0 | 68,565 | 2,713 | [279] | |
| 25 June | 1,025,081 | samples | 259,064 | 25.3 | 53,750 | 13,584 | [280] | |
| 22 June | 90,410,000 | samples | 90,083 | 0.09 | 62,814 | 63 | [281][282][283][284] | |
| 21 June | 604,273 | samples | 68,652 | 11.4 | 12,522 | 1,423 | [285] | |
| 21 June | 35,369 | samples | 2,213 | 6.3 | 7,075 | 443 | [286] | |
| 9 June | 69,115 | cases | 2,247 | 3.3 | 16,956 | 551 | [287] | |
| 21 June | 151,164 | samples | 2,312 | 1.5 | 13,346 | 204 | [288] | |
| 23 May | 99,733 | samples | 927 | 0.93 | 115,530 | 1,074 | [289] | |
| 26 June | 541,212 | samples | 11,038 | 2.0 | 50,609 | 1,032 | [290] | |
| 22 June | 753,812 | cases | 12,727 | 1.7 | 127,137 | 2,147 | [291] | |
| 2 June | 9,508 | 3,195 | 33.6 | 106 | 36 | [292] | ||
| 20 June | 138,880 | samples | 50,183 | 36.1 | 8,129 | 2,937 | [293] | |
| 2 June | 141,002 | samples | 26,384 | 18.7 | 1,409 | 264 | [292] | |
| 21 June | 140,429 | samples | 4,626 | 3.3 | 21,650 | 713 | [294] | |
| 8 June | 90,621 | samples | 1,940 | 2.1 | 68,220 | 1,460 | [295] | |
| 23 June | 223,341 | samples | 4,848 | 2.17 | 1,943 | 42 | [296] | |
| 19 June | 229,843 | samples | 7,142 | 3.1 | 41,464 | 1,288 | [297] | |
| 2 June | 1,493,333 | 142,843 | 9.6 | 22,281 | 2,131 | [298][299][300] | ||
| 9 June | 4,694,147 | samples | 184,861 | 3.9 | 56,454 | 2,223 | [301] | |
| 7 May | 137,924 | 3,091 | 2.2 | 4,439 | 99 | [302] | ||
| 10 May | 98,877 | samples | 4,015 | 4.1 | 9,182 | 373 | [303] | |
| 18 June | 5,465 | samples | 23 | 0.42 | 49,034 | 206 | [304] | |
| 6 May | 7,369 | cases | 1,856 | 25.2 | 561 | 141 | [278] | |
| 8 June | 210,749 | samples | 4,014 | 1.9 | 21,816 | 416 | [305] | |
| 21 June | 69,190 | samples | 1,823 | 2.6 | 189,947 | 5,005 | [306] | |
| 30 June | 8,608,654 | samples | 566,840 | 6.6 | 6,239 | 411 | [307][308] | |
| 28 June | 456,636 | cases | 54,010 | 11.8 | 1,706 | 202 | [309] | |
| 5 June | 1,040,289 | samples | 167,156 | 16.1 | 12,506 | 2,009 | [310] | |
| 7 May | 120,604 | samples | 2,543 | 2.1 | 2,998 | 63 | [311] | |
| 27 June | 419,021 | samples | 25,414 | 6.1 | 85,141 | 5,164 | [312] | |
| 24 June | 886,068 | 21,666 | 2.4 | 96,574 | 2,361 | [313] | ||
| 24 June | 5,107,093 | samples | 239,410 | 4.7 | 84,611 | 3,966 | [314] | |
| 25 May | 18,303 | 2,423 | 13.2 | 694 | 92 | [278] | ||
| 12 May | 7,465 | samples | 507 | 6.8 | 2,739 | 186 | [315] | |
| 17 June | 599,339 | samples | 17,382 | 2.9 | 4,751 | 138 | [316] | |
| 31 May | 812,881 | samples | 10,858 | 1.3 | 43,578 | 582 | [317] | |
| 10 May | 32,097 | samples | 672 | 2.1 | 675 | 14 | [318] | |
| 9 June | 17,199 | samples | 1,269 | 7.4 | 9,500 | 701 | [319] | |
| 5 May | 58,030 | samples | 843 | 1.5 | 9,082 | 132 | [320] | |
| 8 June | 118,322 | samples | 1,088 | 0.92 | 61,627 | 567 | [321] | |
| 12 May | 53,269 | 870 | 1.6 | 7,804 | 127 | [322] | ||
| 2 June | 6,871 | cases | 156 | 2.3 | 1,001 | 23 | [292] | |
| 24 June | 403,841 | samples | 1,804 | 0.45 | 144,522 | 646 | [323] | |
| 29 June | 178,422 | samples | 4,256 | 2.4 | 284,970 | 6,798 | [324] | |
| 2 June | 11,311 | cases | 826 | 7.3 | 431 | 31 | [292] | |
| 21 June | 10,880 | samples | 749 | 6.9 | 569 | 39 | [325] | |
| 16 June | 659,632 | cases | 8,505 | 1.3 | 20,128 | 260 | [326] | |
| 22 May | 17,174 | samples | 1,274 | 7.4 | 43,758 | 3,246 | [327] | |
| 27 May | 64,334 | samples | 612 | 0.95 | 130,347 | 1,240 | [328] | |
| 2 June | 120,358 | samples | 335 | 0.28 | 95,071 | 265 | [329] | |
| 28 June | 483,953 | cases | 212,802 | 44.0 | 3,762 | 1,654 | [330] | |
| 5 May | 8,534 | samples | 324 | 3.8 | 13,520 | 513 | [331] | |
| 2 June | 221,154 | cases | 7,859 | 3.6 | 5,992 | 213 | [332] | |
| 10 May | 3,923 | 91 | 2.3 | 126 | 2.9 | [333] | ||
| 31 May | 24,710 | samples | 224 | 0.91 | 454 | 4.1 | [334] | |
| 24 June | 457,950 | 10,099 | 2.2 | 16,300 | 359 | [335] | ||
| 3 June | 378,912 | cases | 46,942 | 12.4 | 21,745 | 2,694 | [336] | |
| 19 June | 335,167 | samples | 1,159 | 0.35 | 67,254 | 233 | [337] | |
| 7 June | 76,802 | samples | 12,486 | 16.3 | 375 | 61 | [338] | |
| 17 April | 740 | cases | 0 | 0 | 29 | 0 | [339] | |
| 1 June | 30,302 | 2,315 | 7.6 | 14,588 | 1,115 | [340] | ||
| 30 May | 31,346 | 108 | 0.34 | 96,153 | 331 | [341] | ||
| 22 May | 310,635 | cases | 8,745 | 2.8 | 57,872 | 1,629 | [342] | |
| 25 June | 175,278 | samples | 34,902 | 19.9 | 37,715 | 7,510 | [343] | |
| 20 June | 1,239,153 | samples | 202,955 | 16.4 | 5,611 | 919 | [344] | |
| 30 June | 92,206 | samples | 2443 | 2.6 | 18,252 | 484 | [345] | |
| 9 June | 80,720 | 17,233 | 21.3 | 19,325 | 4,126 | [346] | ||
| 19 June | 54,278 | samples | 1,336 | 2.5 | 7,610 | 187 | [347] | |
| 29 June | 1,661,324 | samples | 282,365 | 17.0 | 50,613 | 8,602 | [348] | |
| 28 June | 708,534 | samples | 35,455 | 5.0 | 7,016 | 351 | [349] | |
| 1 June | 931,520 | samples | 23,987 | 2.6 | 24,267 | 625 | [350] | |
| 23 May | 689,705 | samples | 30,471 | 4.4 | 67,114 | 2,965 | [351] | |
| 26 June | 341,914 | cases | 92,784 | 27.1 | 118,677 | 32,205 | [352] | |
| 28 May | 410,000 | samples | 18,791 | 4.6 | 21,132 | 969 | [353] | |
| 28 June | 19,334,442 | samples | 641,156 | 3.3 | 131,755 | 4,369 | [354][355] | |
| 21 June | 111,257 | 728 | 0.65 | 8,590 | 56 | [356] | ||
| 25 May | 898 | 18 | 2.0 | 4,937 | 99 | [357] | ||
| 9 June | 976,815 | 105,283 | 10.8 | 28,058 | 3,024 | [358] | ||
| 11 May | 22,481 | 1,886 | 8.4 | 1,418 | 119 | [359] | ||
| 28 May | 233,479 | cases | 11,300 | 4.8 | 33,528 | 1,623 | [360] | |
| 22 June | 684,359 | samples | 42,313 | 6.2 | 119,987 | 7,419 | [361][362] | |
| 29 June | 208,904 | samples | 1,664 | 0.80 | 38,276 | 305 | [363] | |
| 7 June | 84,684 | samples | 1,495 | 1.8 | 40,440 | 714 | [364] | |
| 6 June | 850,871 | cases | 43,434 | 5.1 | 14,477 | 739 | [365] | |
| 28 June | 1,232,690 | cases | 12,715 | 1.0 | 23,839 | 246 | [366] | |
| 4 June | 4,465,338 | samples | 287,740 | 6.4 | 95,550 | 6,157 | [367][368] | |
| 16 June | 90,010 | cases | 1,915 | 2.1 | 4,154 | 88 | [369] | |
| 2 June | 7,825 | samples | 5,173 | 66.1 | 178 | 118 | [292] | |
| 17 June | 382,804 | cases | 52,189 | 13.6 | 37,066 | 5,053 | [370] | |
| 16 June | 465,722 | samples | 31,146 | 6.7 | 54,099 | 3,618 | [371] | |
| 29 June | 76,491 | cases | 447 | 0.58 | 3,241 | 19 | [372] | |
| 6 June | 2,680 | 509 | 19.0 | 45 | 8.5 | [278] | ||
| 7 May | 89,791 | cases | 2,992 | 3.3 | 1,293 | 43 | [373] | |
| 29 June | 4,969 | samples | 126 | 2.3 | 3,643 | 92 | [374] | |
| 2 June | 52,874 | cases | 1,086 | 2.1 | 4,474 | 92 | [375] | |
| 29 June | 3,331,158 | cases | 198,613 | 6.0 | 40,060 | 2,388 | [376] | |
| 21 June | 166,917 | samples | 770 | 0.46 | 3,649 | 17 | [377] | |
| 23 May | 456,509 | samples | 29,070 | 6.4 | 10,861 | 692 | [378] | |
| 23 June | 3,055,566 | 45,683 | 1.5 | 318,310 | 4,759 | [379] | ||
| 29 June | 9,290,215 | samples | 311,965 | 3.4 | 137,540 | 4,619 | [380] | |
| 28 June | 32,000,928 | samples | 2,596,771 | 8.1 | 96,684 | 7,846 | [381][382] | |
| 19 June | 56,234 | samples | 853 | 1.5 | 16,204 | 246 | [383] | |
| 7 May | 371,000 | samples | 2,298 | 0.62 | 10,900 | 68 | [384] | |
| 20 June | 1,129,176 | samples | 3,790 | 0.34 | 39,089 | 131 | [385] | |
| 15 June | 261,004 | samples | 334 | 0.13 | 2,644 | 3.4 | [386] | |
| 21 June | 55,709 | 463 | 0.83 | 3,748 | 31 | [387] | ||
| ||||||||
References
![]()
- CDC (11 February 2020). "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. Retrieved 9 June 2020.
- Kobokovich A, West R, Gronvall G. "Global Progress on COVID-19 Serology-Based Testing". Johns Hopkins Center for Health Security. Retrieved 9 June 2020.
- "Test for Past Infection – CDC". CDC.gov. Centers for Disease Control and Prevention. 11 February 2020. Archived from the original on 16 May 2020. Retrieved 19 May 2020.
Antibody blood tests, also called antibody tests, check your blood by looking for antibodies, which show if you had a previous infection with the virus. Depending on when someone was infected and the timing of the test, the test may not find antibodies in someone with a current COVID-19 infection.
- Abbasi J (April 2020). "The Promise and Peril of Antibody Testing for COVID-19". Jama. JAMA Network. 323 (19): 1881. doi:10.1001/jama.2020.6170. PMID 32301958. Retrieved 20 April 2020.
- Brotschi M (7 March 2020). "Bund sucht nicht mehr alle Corona-Infizierten". Der Bund (in German). ISSN 0774-6156. Retrieved 9 June 2020.
- Van Beusekom, Mary (24 March 2020). "Italian doctors note high COVID-19 death rate, urge action". CIDRAP News. Retrieved 9 June 2020.
- Otmani M (22 March 2020). "COVID-19: First results of the voluntary screening in Iceland". Nordic Life Science – the leading Nordic life science news service. Retrieved 9 June 2020.
- Ward, D. (April 2020) "Sampling Bias: Explaining Wide Variations in COVID-19 Case Fatality Rates". WardEnvironment. doi: 10.13140/RG.2.2.24953.62564/1
- Henriques M (2 April 2020). "Coronavirus: Why death and mortality rates differ". BBC News. Retrieved 9 June 2020.
- Ward D (May 2020). Sampling Bias: Explaining Variations in Age Distributions of COVID-19 Cases. Technical Report (Report). WardEnvironment. doi:10.13140/RG.2.2.27321.19047/2.
- "Siouxsie Wiles & Toby Morris: What we don't know about Covid-19". The Spinoff. 6 May 2020. Retrieved 6 May 2020.
- "Testing for COVID-19 – CDC". CDC.gov. Centers for Disease Control and Prevention. 20 May 2020. Archived from the original on 19 May 2020. Retrieved 20 May 2020.
Two kinds of tests are available for COVID-19: viral tests and antibody tests.
- "RNA Extraction". AssayGenie. Retrieved 7 May 2020.
- "How is the COVID-19 Virus Detected using Real Time RT-PCR?". IAEA. 27 March 2020. Retrieved 5 May 2020.
- "Curetis Group Company Ares Genetics and BGI Group Collaborate to Offer Next-Generation Sequencing and PCR-based Coronavirus (2019-nCoV) Testing in Europe". GlobeNewswire News Room. 30 January 2020. Archived from the original on 31 January 2020. Retrieved 1 February 2020.
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (April 2009). "The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments". Clinical Chemistry. 55 (4): 611–22. doi:10.1373/clinchem.2008.112797. PMID 19246619.
- "Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis" (PDF). Clinical Science. 23 September 2005. Retrieved 5 May 2020.
- "The Basics: RT-PCR". ThermoFisher Scientific. Retrieved 5 May 2020.
- Kang XP, Jiang T, Li YQ, Lin F, Liu H, Chang GH, et al. (June 2010). "A duplex real-time RT-PCR assay for detecting H5N1 avian influenza virus and pandemic H1N1 influenza virus". Virology Journal. 7: 113. doi:10.1186/1743-422X-7-113. PMC 2892456. PMID 20515509.
- Joyce C (2002). Quantitative RT-PCR. A review of current methodologies. Methods Mol. Biol. 193. pp. 83–92. doi:10.1385/1-59259-283-X:083. ISBN 978-1-59259-283-8. PMID 12325527.
- Varkonyi-Gasic E, Hellens RP (2010). "qRT-PCR of Small RNAs". Plant Epigenetics. Methods in Molecular Biology. 631. pp. 109–22. doi:10.1007/978-1-60761-646-7_10. ISBN 978-1-60761-645-0. PMID 20204872.
- "Accelerated Emergency Use Authorization (Eua) Summary Covid-19 Rt-Pcr Test (Laboratory Corporation of America)". FDA. Retrieved 3 April 2020.
- Taylor S, Wakem M, Dijkman G, Alsarraj M, Nguyen M (April 2010). "A practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelines". Methods. 50 (4): S1-5. doi:10.1016/j.ymeth.2010.01.005. PMID 20215014.
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. (April 2009). "The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments". Clinical Chemistry. 55 (4): 611–22. doi:10.1373/clinchem.2008.112797. PMID 19246619.
- "Real-Time RT-PCR Panel for Detection 2019-nCoV". Centers for Disease Control and Prevention. 29 January 2020. Archived from the original on 30 January 2020. Retrieved 1 February 2020.
- Drosten C (26 March 2020). "Coronavirus-Update Folge 22" (PDF). NDR. Archived (PDF) from the original on 31 March 2020. Retrieved 2 April 2020.
- "Here's where things stand on COVID-19 tests in the U.S." ScienceNews. 17 April 2020. Retrieved 6 May 2020.
- Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q (April 2020). "Saliva: potential diagnostic value and transmission of 2019-nCoV". International Journal of Oral Science. 12 (1): 11. doi:10.1038/s41368-020-0080-z. PMID 32300101.
- Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, et al. (May 2003). "Identification of a novel coronavirus in patients with severe acute respiratory syndrome". The New England Journal of Medicine. 348 (20): 1967–76. doi:10.1056/NEJMoa030747. PMID 12690091.
- "COVID-19 saliva tests: What is the benefit?". Mayo Clinic. 16 April 2020. Retrieved 6 May 2020.
- "New Rutgers Saliva Test for Coronavirus Gets FDA Approval". Rutgers.edu. 13 April 2020. Retrieved 1 May 2020.
- "FDA authorizes Covid-19 saliva test for emergency use". CNN. 14 April 2020. Retrieved 1 May 2020.
- "Yale University School of Public Health finds saliva samples promising alternative to nasopharyngeal swab". Merck Manual. 29 April 2020. Retrieved 6 April 2020.
- Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- "Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States". Nature Medicine. 26 (6): 861–868. June 2020. doi:10.1038/s41591-020-0877-5. PMID 32327757.
- Young BE, Ong SW, Kalimuddin S, Low JG, Tan SY, Loh J, et al. (March 2020). "Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore". Jama. 323 (15): 1488. doi:10.1001/jama.2020.3204. PMC 7054855. PMID 32125362.
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. (March 2020). "SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients". The New England Journal of Medicine. 382 (12): 1177–1179. doi:10.1056/NEJMc2001737. PMC 7121626. PMID 32074444.
- Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. (May 2020). "Virological assessment of hospitalized patients with COVID-2019". Nature. 581 (7809): 465–469. doi:10.1038/s41586-020-2196-x. PMID 32235945.
- Zimmer C (5 May 2020). "With Crispr, a Possible Quick Test for the Coronavirus". The New York Times. ISSN 0362-4331. Retrieved 14 May 2020.
- "STOPCovid". stopcovid.science. Retrieved 14 June 2020.
- Joung J, Ladha A, Saito M, Segel M, Bruneau R, Huang MW, et al. (May 2020). "Point-of-care testing for COVID-19 using SHERLOCK diagnostics". medRxiv: 2020.05.04.20091231. doi:10.1101/2020.05.04.20091231. PMID 32511521.
- "Developing Antibodies and Antigens for COVID-19 Diagnostics". Technology Networks. 6 April 2020. Retrieved 30 April 2020.
- "NIH launches competition to speed COVID-19 diagnostics". AAAS. 29 April 2020. Retrieved 1 May 2020.
- "Remarks by President Trump, Vice President Pence, and Members of the Coronavirus Task Force in Press Briefing April 17, 2020". Whitehouse.gov. 17 April 2020. Retrieved 30 April 2020.
- "What to know about the three main types of coronavirus tests". CNN. 29 April 2020. Retrieved 30 April 2020.
- Office of the Commissioner (9 May 2020). "Coronavirus (COVID-19) Update: FDA Authorizes First Antigen Test to Help in the Rapid Detection of the Virus that Causes COVID-19 in Patients". FDA.
- Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (March 2020). "Coronavirus Disease 2019 (COVID-19): A Systematic Review of Imaging Findings in 919 Patients". AJR. American Journal of Roentgenology: 1–7. doi:10.2214/AJR.20.23034. PMID 32174129.
Known features of COVID-19 on initial CT include bilateral multilobar ground-glass opacification (GGO) with a peripheral or posterior distribution, mainly in the lower lobes and less frequently within the right middle lobe.
- Lee EY, Ng MY, Khong PL (April 2020). "COVID-19 pneumonia: what has CT taught us?". The Lancet. Infectious Diseases. 20 (4): 384–385. doi:10.1016/S1473-3099(20)30134-1. PMC 7128449. PMID 32105641.
- "ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection". American College of Radiology. 22 March 2020. Archived from the original on 13 May 2020. Retrieved 20 May 2020.
- "The next frontier in coronavirus testing: Identifying the full scope of the pandemic, not just individual infections". STAT. 27 March 2020. Retrieved 30 April 2020.
- "Cellex Emergency Use Authorization". FDA. 1 April 2020. Retrieved 10 April 2020.
- "Will an Antibody Test Allow Us to Go Back to School or Work?". New York Times. 10 April 2020. Retrieved 15 April 2020.
- "Mount Sinai Emergency Use Authorization". FDA. 15 April 2020. Retrieved 18 April 2020.
- "What Immunity to COVID-19 Really Means". Scientific American. 10 April 2020. Archived from the original on 28 April 2020.
- "Global Progress on COVID-19 Serology-Based Testing". Johns Hopkins Center for Health Security. Retrieved 14 June 2020.
- "A SARS-CoV-2 surrogate virus neutralization test (sVNT) based on antibody-mediated blockage of ACE2-spike (RBD) protein-protein interaction". Research Square. 23 April 2020. Retrieved 28 April 2020.
- "Will antibody tests for the coronavirus really change everything?". Nature. 18 April 2020. Retrieved 20 April 2020.
- "Q&A on COVID-19 Antibody Tests". factcheck.org. 27 April 2020. Retrieved 28 April 2020.
- "Neutralising antibody". Biology-Online. 2008. Retrieved 4 July 2009.
- Schmaljohn AL (July 2013). "Protective antiviral antibodies that lack neutralizing activity: precedents and evolution of concepts". Current HIV Research. 11 (5): 345–53. doi:10.2174/1570162x113116660057. PMID 24191933.
- "Virus neutralization by antibodies". Virology Blog. 24 July 2009. Retrieved 29 April 2020.
- "expert reaction to announcement by Roche of its new serology test for COVID-19 antibodies". Science Media Centre. 17 April 2020. Retrieved 28 April 2020.
- Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH (September 2007). "Disappearance of antibodies to SARS-associated coronavirus after recovery". The New England Journal of Medicine. NEJM. 357 (11): 1162–3. doi:10.1056/NEJMc070348. PMID 17855683.
- "Lack of Peripheral Memory B Cell Responses in Recovered Patients with Severe Acute Respiratory Syndrome: A Six-Year Follow-Up Study" (PDF). Journal of Immunology. 19 April 2011. Retrieved 1 May 2020.
- Leslie M (May 2020). "T cells found in coronavirus patients 'bode well' for long-term immunity". Science. 368 (6493): 809–810. doi:10.1126/science.368.6493.809. PMID 32439770.
- Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Young et al. (2020)
- Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Midgley et al. (2020)
- "What Is R0? Gauging Contagious Infections". Healthline.
- He X, Lau EH, Wu P, Deng X, Wang J, Hao X, et al. (May 2020). "Temporal dynamics in viral shedding and transmissibility of COVID-19". Nature Medicine. 26 (5): 672–675. doi:10.1038/s41591-020-0869-5. PMID 32296168.
- "Coronavirus Disease 2019 (COVID-19)". Centers for Disease Control and Prevention. 29 April 2020. Retrieved 23 May 2020.
- Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, et al. (June 2009). "Pandemic potential of a strain of influenza A (H1N1): early findings". Science. 324 (5934): 1557–61. Bibcode:2009Sci...324.1557F. doi:10.1126/science.1176062. PMC 3735127. PMID 19433588.Free text
- "COVID-19 Infections Tracker". COVID-19 Projections Using Machine Learning. Retrieved 11 May 2020.
- "About covid19-projections.com". COVID-19 Projections Using Machine Learning. Retrieved 11 May 2020.
- "People with COVID-19 may be infectious days before symptoms: study". medicalxpress.com. 15 April 2020. Retrieved 11 May 2020.
- "50 Percent of People with COVID-19 Aren't Aware They Have Virus". Healthline. 24 April 2020. Retrieved 11 May 2020.
- "Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19". CDC. 3 May 2020. Archived from the original on 4 May 2020.
- Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Wölfel et al. (2020)
- Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. (May 2020). "Presymptomatic SARS-CoV-2 Infections and Transmission in a Skilled Nursing Facility". The New England Journal of Medicine. 382 (22): 2081–2090. doi:10.1056/NEJMoa2008457. PMC 7200056. PMID 32329971.
- Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced
- CDC unpublished data
- Midgley et al. (2020)
- Wölfel et al. (2020)
- Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19 (2020) referenced Xiao AT, Tong YX, Zhang S (April 2020). "Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients". Clinical Infectious Diseases. doi:10.1093/cid/ciaa460. PMC 7188124. PMID 32306036.
- Roser M, Ritchie H, Ortiz-Ospina E (4 March 2020). "Coronavirus Disease (COVID-19) – Statistics and Research". Our World in Data – via ourworldindata.org.
- "Laboratory Readiness for Detecting the 2019 novel coronavirus (2019-nCoV) infection in Malaysia". Director-General of Health, Malaysia. 9 February 2020.
- "Malaysia must ramp up testing". The Star Malaysia. 26 March 2020.
- "BGI Sequencer, Coronavirus Molecular Assays Granted Emergency Use Approval in China". GenomeWeb. Retrieved 9 March 2020.
- "UK defends coronavirus response after Reuters investigation". Reuters. 9 April 2020. Retrieved 12 April 2020.
After developing a test for the new virus by Jan. 10
- "COVID-19 virus testing in NHS laboratories" (PDF). NHS England and NHS Improvement. 16 March 2020.
- "PHE novel coronavirus diagnostic test rolled out across UK". GOV.UK. Retrieved 30 March 2020; "'Increased likelihood' of China virus reaching UK". BBC News. 23 January 2020. Retrieved 30 March 2020; "PHE tells patients with suspected coronavirus to call GP or NHS 111". The Pharmaceutical Journal. 27 January 2020. Retrieved 30 March 2020.
- "日本が韓国の新型コロナウイルス対策から学べること──(1)検査体制". Newsweek Japan (in Japanese). 2 April 2020. Retrieved 5 June 2020.
- Sheridan C (April 2020). "Coronavirus and the race to distribute reliable diagnostics". Nature Biotechnology. 38 (4): 382–384. doi:10.1038/d41587-020-00002-2. PMID 32265548.
- "CDC Diagnostic Test for COVID-19". CDC.gov. Retrieved 15 June 2019.
- "PolitiFact – Biden falsely says Trump administration rejected WHO coronavirus test kits (that were never offered)". @politifact.
- Совещание по вопросам развития ситуации с коронавирусной инфекцией и мерам по её профилактике
- Transcript for the CDC Telebriefing Update on COVID-19, 28 February 2020
- Jeong S (28 February 2020). "Korea approves 2 more COVID-19 detection kits for urgent use". Korea Biomedical Review. Retrieved 12 March 2020.
- "Wuhan Test Lab Opens; CDC Ships Diagnostic Kits: Virus Update". Bloomberg. 5 February 2020. Retrieved 7 February 2020.
- "China virus crisis deepens as whistleblower doctor dies". AFP.com. 27 February 2012. Retrieved 7 February 2020.
- 日检测量达万份的"火眼"实验室连夜试运行.
- "BGI's Coronavirus Response? Build a Lab in Wuhan". GEN – Genetic Engineering and Biotechnology News. 12 February 2020. Retrieved 27 March 2020.
- "В России зарегистрирована отечественная тест-система для определения коронавируса". Interfax-Russia.ru. 14 February 2020.
- "COVID-19 Local Laboratory Solution". BGI – Global. Retrieved 27 March 2020.
- "'Test, Test, Test': WHO Chief's Coronavirus Message to World". The New York Times. 16 March 2020. Retrieved 16 March 2020.
- "'Test, test, test': WHO calls for more coronavirus testing – video". The Guardian. 16 March 2020. Retrieved 16 March 2020.
- "Coronavirus disease 2019 (COVID-19) pandemic: increased transmission in the EU/EEA and the UK –seventh update" (PDF). European Centre for Disease Prevention and Control. 25 March 2020. pp. 15–16. Retrieved 29 March 2020.
the current shortages of laboratory consumables and reagents affect diagnostic capacity and hamper the epidemic response at the national and local levels. The laboratories have experienced delayed or missing deliveries of swabbing material, plastic consumables, RNA extraction and RT-PCR reagents, and PPE. This is affecting laboratories in all EU/EEA countries.
- Baird RP (24 March 2020). "Why Widespread Coronavirus Testing Isn't Coming Anytime Soon". The New Yorker. Archived from the original on 28 March 2020. Retrieved 29 March 2020.
South Dakota, said that her state's public-health laboratory—the only lab doing COVID-19 testing in the state—had so much trouble securing reagents that it was forced to temporarily stop testing altogether. also noted critical shortages of extraction kits, reagents, and test kits
- Ossola A (25 March 2020). "Here are the coronavirus testing materials that are in short supply in the US". Quartz. Archived from the original on 26 March 2020. Retrieved 29 March 2020.
extract the virus's genetic material—in this case, RNA—using a set of chemicals that usually come in pre-assembled kits. 'The big shortage is extraction kits' There are no easy replacements here: 'These reagents that are used in extraction are fairly complex chemicals. They have to be very pure, and they have to be in pure solution'
- Plumbo G. "Mayo Clinic develops test to detect COVID-19 infection". Mayo Clinic. Retrieved 13 March 2020.
- Chen, Lulu Yilun (16 March 2020). "Heartbreak in the Streets of Wuhan". Bloomberg BusinessWeek. Retrieved 23 May 2020.
- Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W (March 2020). "Detection of SARS-CoV-2 in Different Types of Clinical Specimens". Jama. 323 (18): 1843–1844. doi:10.1001/jama.2020.3786. PMC 7066521. PMID 32159775.
- "Comparing RT-PCR and Chest CT for Diagnosing COVID-19". HCPLive®. Retrieved 23 May 2020.
- "LabCorp Launches Test for Coronavirus Disease 2019 (COVID-19) | Laboratory Corporation of America Holdings". ir.labcorp.com. Retrieved 16 June 2020.
- "Covid19 : COVID-19". questdiagnostics.com.
- Stein R (3 April 2020). "Coronavirus Testing Backlogs Continue As Laboratories Struggle To Keep Up With Demand". NPR.org. Retrieved 15 June 2020.
- Mandavilli A, Edmondson C (25 May 2020). "'This Is Not the Hunger Games': National Testing Strategy Draws Concerns". The New York Times.
- "Hologic's Molecular Test for the Novel Coronavirus, SARS-CoV-2, Receives FDA Emergency Use Authorization". Hologic. 16 March 2020. Retrieved 13 April 2020.
- "Emergency Use Authorization". FDA. 30 April 2020. Archived from the original on 29 April 2020.
- "FDA Approves Abbott Laboratories Coronavirus Test, Company To Ship 150,000 Kits". IBTimes.com. 19 March 2020. Archived from the original on 20 March 2020.
- "Sunnyvale company wins FDA approval for first rapid coronavirus test with 45-minute detection time". EastBayTimes.com. 21 March 2020. Archived from the original on 22 March 2020.
- "Xpert® Xpress SARS-CoV-2 has received FDA Emergency Use Authorization". cepheid.com. Retrieved 13 April 2020.
- "Letter from FDA". FDA. 27 March 2020. Retrieved 2 April 2020.
- Sullivan H, Rawlinson K, Gayle D, Topping A, Mohdin A, Willsher K, Wintour P, Wearden G, Greenfield P (31 March 2020). "Global confirmed virus death toll passes 40,000 – as it happened". The Guardian. ISSN 0261-3077. Retrieved 1 April 2020.
- "VIDEO: UAE sets up COVID-19 detection lab in just 14 days". Gulf Today. 31 March 2020.
- "Daily COVID-19 tests per thousand people". Our World in Data. Retrieved 15 April 2020.
- "Total tests for COVID-19 per 1,000 people". Our World in Data. Retrieved 15 April 2020.
- "Covid-19 – Tests auf das Coronavirus: Wann, wo und wie?". Deutschlandfunk (in German). 19 March 2020. Retrieved 24 March 2020.
- Charisius H (26 March 2020). "Covid-19: Wie gut testet Deutschland?" (in German). Retrieved 26 March 2020.
- "Coronavirus Disease 2019 Daily Situation Report of the Robert Koch Institute" (PDF). Robert Koch Institute. 26 March 2020. Retrieved 28 April 2020.
- "80% of Rapid COVID-19 Tests the Czech Republic Bought From China are Wrong". Prague Morning. 26 March 2020.
- Blažek V (23 March 2020). "Úřad dopředu psal, kdy mohou rychlotesty selhat. I tak je stát nasadil". Zeznam Zprávy (in Czech). Retrieved 7 April 2020.
Indeed, the rapid tests that arrived from China a few days ago do not really reliably detect the infection at an early stage.
- DUDIK, ANDREA; TOMEK, RADOSLAV (1 April 2020). "Europe turned to China for coronavirus testing help. Why some are now regretting it". Fortune. Retrieved 28 June 2020.
- "Faulty virus tests cloud China's European outreach over COVID-19". The Jakarta Post. Retrieved 23 May 2020.
- "Coronavirus test kits purchased from China are unreliable, says Science Committee member". www.duvarenglish.com. 27 March 2020. Retrieved 28 June 2020.
- Soylu, Ragip (27 March 2020). "Coronavirus: Turkey rejects Chinese testing kits over inaccurate results". Middle East Eye. Retrieved 28 June 2020.
- Weber N, Rydlink K, Irene Berres (5 March 2020). "Coronavirus und Covid-19: So testet Deutschland". Der Spiegel (in German). Retrieved 23 March 2020.
- Oltermann P (22 March 2020). "Germany's low coronavirus mortality rate intrigues experts". The Guardian. ISSN 0261-3077. Retrieved 24 March 2020.
- "Chinese firm to replace exported coronavirus test kits deemed defective by Spain". 27 March 2020 – via www.reuters.com.
- "Coronavirus Pandemic Data Explorer". Our World in Data. Retrieved 28 June 2020.
- Romano, Andrew. (7 April 2020). "Fauci once dismissed concerns about 'silent carriers' of coronavirus. Not anymore." Yahoo News Retrieved 17 April 2020.
- "WHO lists two COVID-19 tests for emergency use". World Health Organization. Retrieved 10 April 2020.
- "Health Canada approves new rapid COVID-testing kits". The Globe and Mail Inc. 13 April 2020.
- "The power of DNA testing for everyone". Spartan Bioscience. Archived from the original on 22 April 2020. Retrieved 14 April 2020.
- "Coronavirus (COVID-19): Scaling up our testing programmes" (PDF). Department of Health and Social Care. 4 April 2020.
- "NHS pilots home testing for coronavirus". MobiHealthNews. 24 February 2020. Archived from the original on 25 February 2020.
- Kenber B (6 April 2020). Smyth C, Kennedy D (eds.). "Britain has millions of coronavirus antibody tests, but they don't work" – via www.thetimes.co.uk.
None of the antibody tests ordered by the government is good enough to use, the new testing chief has admitted. Professor John Newton said that tests ordered from China.
- "Government's testing chief admits none of 3.5m coronavirus antibody kits work sufficiently". The Independent. 6 April 2020.
- "ICMR asks states not to use rapid test kits for two days". The Telegraph. 21 April 2020.
- Vogel G (21 April 2020). "Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable". Science | AAAS. Retrieved 31 May 2020.
- "WHO Director-General's opening remarks at the media briefing on COVID-19 - 20 April 2020". www.who.int. 20 April 2020. Retrieved 31 May 2020.
- "MLB antibody study: 0.7% of those tested had been exposed to coronavirus". SFChronicle.com. 11 May 2020. Archived from the original on 19 May 2020. Retrieved 13 May 2020.
- Wagner J (15 April 2020). "M.L.B. Employees Become the Subjects of a Huge Coronavirus Study". The New York Times. Retrieved 20 May 2020.
- "Fewer than 1% of MLB employees test positive for COVID-19 antibodies". Los Angeles Times. 10 May 2020. Retrieved 20 May 2020.
- "US Historical Data". The COVID Tracking Project.
- "COVID-19: Tests per day". Our World in Data. Retrieved 15 April 2020.
- "Roche's COVID-19 antibody test receives FDA Emergency Use Authorization and is available in markets accepting the CE mark". Roche (Press release). 3 May 2020. Retrieved 8 May 2020.
- "Roche Diagnostics GmbH Elecsys Anti-SARS-CoV-2" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 8 May 2020.
- "FDA issues emergency approval of new antigen test that is cheaper, faster and simpler". Washington Post. 9 May 2020.
- Commissioner, Office of the (14 May 2020). "Coronavirus (COVID-19) Update: FDA Informs Public About Possible Accuracy Concerns with Abbott ID NOW Point-of-Care Test". FDA. Retrieved 15 May 2020.
- Ho D (21 May 2020). "Israel's Ben-Gurion University develops one-minute coronavirus test". BioWorld.com. Retrieved 7 June 2020.
- Mannix L (13 May 2020). "'Flipping a coin': COVID-19 antibody tests 'should not be used'". The Sydney Morning Herald. Retrieved 23 May 2020.
- Mannix L (12 May 2020). "The government bought 1.5 million antibody tests. They can't be used". The Sydney Morning Herald. Retrieved 29 May 2020.
- Australian Government Department of Health Therapeutic Goods Administration (22 May 2020). "Post-market evaluation of serology-based point of care tests". Therapeutic Goods Administration (TGA). Retrieved 23 May 2020.
- "HGHI and NPR publish new state testing targets – Pandemics Explained". globalepidemics.org. 7 May 2020. Retrieved 11 May 2020.
- "U.S. Coronavirus Testing Still Falls Short. How's Your State Doing?". NPR.org. 7 May 2020. Retrieved 11 May 2020.
- "What Testing Capacity Do We Need?". The Henry J. Kaiser Family Foundation. 17 April 2020. Retrieved 11 May 2020.
- Romer P. "Roadmap to responsibly reopen America" (PDF). Retrieved 11 May 2020.
- "Roadmap to pandemic resilience" (PDF). EDMOND J. SAFRA CENTER FOR ETHICS AT HARVARD UNIVERSITY. 20 April 2020. Retrieved 11 May 2020.
- "National Covid-19 Testing Action Plan". The Rockefeller Foundation. Retrieved 11 May 2020.
- Tuzman, Karen Tkach; Jun 11, Associate Editor |; Gmt, 2020 | 5:29 Pm. "Emerging COVID-19 serology tests aim for neutralizing antibodies". BioCentury. Retrieved 26 June 2020.
- Lamb LE, Bartolone SN, Ward E, Chancellor MB (12 June 2020). "Rapid detection of novel coronavirus/Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by reverse transcription-loop-mediated isothermal amplification". PloS One. 15 (6): e0234682. doi:10.1371/journal.pone.0234682. PMC 7292379. PMID 32530929.
- "All State View of Week to Week Change in Percentage of Positive Tests". Johns Hopkins Coronavirus Resource Center. 15 June 2020. Retrieved 15 June 2020.
- jkiger@postbulletin.com, Jeff Kiger. "Mayo Clinic starts drive-thru testing for COVID-19". PostBulletin.com. Retrieved 13 March 2020.
- Hawkins, Andrew J. (11 March 2020). "Some states are offering drive-thru coronavirus testing". The Verge. Retrieved 13 March 2020.
- "South Korea's Drive-Through Testing For Coronavirus Is Fast – And Free". npr. 11 March 2020. Retrieved 16 March 2020.
- "In Age of COVID-19, Hong Kong Innovates To Test And Quarantine Thousands". NPR.org.
- "Pooling method allows dozens of COVID-19 tests to run simultaneously". medicalxpress.com. Retrieved 24 March 2020.
- "Israeli team has coronavirus test kit to test dozens of people at once". The Jerusalem Post | JPost.com. Retrieved 24 March 2020.
- Israel21c Staff (19 March 2020). "Israelis introduce method for accelerated COVID-19 testing". Israel21c. Retrieved 24 March 2020.
- "[Coronavirus] Verified 'sample pooling' introduced to prevent herd infection in S. Korea". ajudaily.com. 9 April 2020. Retrieved 19 April 2020.
- "Gov. Ricketts provides update on coronavirus testing". KMTV. 24 March 2020. Retrieved 19 April 2020.
- May 2020, Nicoletta Lanese-Staff Writer 28. "Wuhan tested millions of people for COVID-19 in just days. Could US cities do the same?". livescience.com. Retrieved 28 June 2020.
- "Latest coronavirus update: UP to begin 'pool testing' of Covid suspects". Free Press Journal. Retrieved 19 April 2020.
- Sumati Yengkhom. "West Bengal to start pool testing of samples in low-risk zones". The Times of India. Retrieved 19 April 2020.
- "Punjab launches pool testing". Retrieved 19 April 2020.
- "'Chhattisgarh to adopt pool sample testing': Health minister TS Singh Deo on Covid-19". Hindustan Times. 15 April 2020. Retrieved 19 April 2020.
- "Maharashtra to go for pool testing to defeat coronavirus". Deccan Herald. 12 April 2020. Retrieved 19 April 2020.
- "Origami Assays". Origami Assays. 2 April 2020. Retrieved 7 April 2020.
- Pulia, Michael S.; O'Brien, Terrence P.; Hou, Peter C.; Schuman, Andrew; Sambursky, Robert (August 2020). "Multi-tiered screening and diagnosis strategy for COVID-19: a model for sustainable testing capacity in response to pandemic". Annals of Medicine. 52 (5): 207–214. doi:10.1080/07853890.2020.1763449. ISSN 1365-2060. PMID 32370561.
- "Which States Are Doing Enough Testing? This Benchmark Helps Settle The Debate". NPR.org. 22 April 2020. Retrieved 11 May 2020.
- "ROADMAP TO PANDEMIC RESILIENCE" (PDF). Edmond J. Safra Center for Ethics. 20 April 2020. Retrieved 19 May 2020.
- "Certified Service Providers". Pacific Biosciences. Retrieved 18 May 2020.
- "Service Provider Program – US". www.thermofisher.com. ThermoFisher Scientific. Retrieved 18 May 2020.
- "Paul Romer". paulromer.net. Simulating Covid-19: Part 2. Retrieved 19 May 2020.
- Lee, Timothy B. (2 April 2020). "America's COVID-19 testing has stalled, and that's a big problem". Ars Technica.
- "[NEW PRODUCT] COVID-19 KIT". kogene.co.kr. 27 February 2020. Archived from the original on 23 April 2020.
- ID NOW COVID-19, Instruction for Use, FDA
- "The scramble for the rapid coronavirus tests everybody wants". Washington Post. 1 April 2020.
- Sofia 2 SARS Antigen FIA Instructions for Use, FDA.gov
- "NIH Begins Study to Quantify Undetected Cases of Coronavirus Infection | NIH: National Institute of Allergy and Infectious Diseases". niaid.nih.gov. Retrieved 11 April 2020.
- Mandavilli A, Thomas K (10 April 2020). "Will an Antibody Test Allow Us to Go Back to School or Work?". The New York Times. Retrieved 11 April 2020.
- "Quest Diagnostics Launches Consumer-Initiated COVID-19 Antibody Test Through QuestDirect™". Quest Diagnosics. 28 April 2020.
- Fellmann F. (March 2020). (in German) "Jetzt beginnt die Suche nach den Genesenen". Tages Anzeiger. Retrieved 28 March 2020.
- "EUA Authorized Serology Test Performance". U.S. Food and Drug Administration (FDA). 7 May 2020. Retrieved 8 May 2020.
- Ferran, Maureen (7 May 2020). "COVID-19 tests are far from perfect, but accuracy isn't the biggest problem". Popular Science. Retrieved 10 May 2020.
- "Serological testing for SARS-CoV-2 antibodies". American Medical Association. 14 May 2020. Retrieved 29 May 2020.
- "Interim Guidelines for COVID-19 Antibody Testing". Centers for Disease COntrol and Prevention. 23 May 2020. Retrieved 29 May 2020.
- Ferran, Maureen. "Coronavirus tests are pretty accurate, but far from perfect". The Conversation. Retrieved 23 May 2020.
- "Comparing RT-PCR and Chest CT for Diagnosing COVID-19". HCPLive®. Retrieved 23 May 2020.
- "Symptom-Based Strategy to Discontinue Isolation for Persons with COVID-19". Centers for Disease Control and Prevention. 30 April 2020.
- Xiao AT, Tong YX, Zhang S (April 2020). "Profile of RT-PCR for SARS-CoV-2: a preliminary study from 56 COVID-19 patients". Clinical Infectious Diseases. doi:10.1093/cid/ciaa460. PMC 7188124. PMID 32306036.
- van Kasteren PB, van der Veer B, van den Brink S, Wijsman L, de Jonge J, van den Brandt A, et al. (July 2020). "Comparison of seven commercial RT-PCR diagnostic kits for COVID-19". Journal of Clinical Virology. 128: 104412. doi:10.1016/j.jcv.2020.104412. PMC 7206434. PMID 32416600.
- "Chinese Covid-19 test kit outstrips alternatives in Dutch study". South China Morning Post. 20 May 2020. Retrieved 23 May 2020.
- "Study Raises Questions About False Negatives From Quick COVID-19 Test". NPR. 21 April 2020. Retrieved 1 May 2020.
- Thomas, Katie (13 May 2020). "Coronavirus Testing Used by the White House Could Miss Infections". The New York Times. ISSN 0362-4331. Retrieved 14 May 2020.
- "National laboratories". who.int.
- "PHE novel coronavirus diagnostic test rolled out across UK". GOV.UK. Retrieved 12 April 2020.
In addition to processing samples from suspected cases in this country, PHE is now working as a reference laboratory for WHO, testing samples from countries that do not have assured testing capabilities.
- "Specimen referral for COVID-19 – operational details of WHO reference laboratories providing confirmatory testing for COVID-19" (PDF). World Health Organization. Retrieved 29 March 2020.
- "COVID-19: First results of the voluntary screening in Iceland". Nordic Life Science – the leading Nordic life science news service. 27 March 2020. Retrieved 5 April 2020.
- "How an experiment helped one Italian town find 'submerged infections,' cut new COVID-19 cases to zero". National Post. 19 March 2020.
- = 5 "PCR拡充が必要 専門家会議が会見 (全文1) page5" Check
|url=value (help). THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. Retrieved 27 May 2020. - "「新型コロナウイルス感染拡大阻止 最前線からの報告". NHK (in Japanese). 15 April 2020. Retrieved 27 May 2020.
- "Did Japan Just Beat the Virus Without Lockdowns or Mass Testing?". Bloomberg. 23 May 2020. Retrieved 27 May 2020.
- = 3 "PCR拡充が必要 専門家会議が会見 (全文1) page3" Check
|url=value (help). THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. Retrieved 27 May 2020. - "新型コロナウイルス 感染爆発をどう防ぐか". NHK (in Japanese). 8 April 2020. Retrieved 27 May 2020.
- "第1波は終息するも欧米からの帰国者経由の第2波が拡大". 日経メディカル (in Japanese). 12 May 2020. Retrieved 27 May 2020.
- "専門家に聞く"新型コロナウイルス"との闘い方と対策". NHK (in Japanese). 27 March 2020. Retrieved 27 May 2020.
- "新型コロナ抗原検査キット、13日から実用化 加藤厚労相が発表 PCRとの併用を想定". 毎日新聞 (in Japanese). 12 May 2020. Retrieved 27 May 2020.
- "コロナ抗原検査が使用可能に、陽性のみ確定診断". 日経メディカル (in Japanese). 12 May 2020. Retrieved 15 May 2020.
- = 4 "PCR拡充が必要 専門家会議が会見 (全文1) page4" Check
|url=value (help). THE PAGE (in Japanese). Yahoo!ニュース. 5 May 2020. Retrieved 27 May 2020. - "クルーズ船112人治療で「院内感染」ゼロ!「自衛隊中央病院」はなぜ奇跡を起こせたのか". 週刊新潮 (in Japanese). 30 April 2020. Retrieved 27 May 2020.
- "「PCR検査数少ないが、死亡者数・率低い」専門家会議". m3.com (in Japanese). 5 May 2020. Retrieved 27 May 2020.
- "クルーズ船112人治療で「院内感染」ゼロ!「自衛隊中央病院」はなぜ奇跡を起こせたのか". 週刊新潮 (in Japanese). 30 April 2020. Retrieved 28 May 2020.
- "調査報告クルーズ船 ウイルス対策のカギは?". NHK (in Japanese). 7 May 2020. Retrieved 24 May 2020.
- "Over 3 mln COVID-19 tests conducted in Russia". TASS. 27 April 2020. Archived from the original on 11 May 2020. Retrieved 29 April 2020.
- "Popova said explosive growth in incidence was not allowed due to measures taken". TASS. 28 April 2020. Retrieved 29 April 2020.
- "COVID-19 outbreak: Petition to close schools in Singapore garners 7,700 signatures to date". msn.com.
- Kuhn, Anthony (12 March 2020). "Experts Credit South Korea's Extensive Testing For Curbing Coronavirus Spread". NPR.org. Retrieved 28 June 2020.
- "日本が韓国の新型コロナウイルス対策から学べること──(3)情報公開". Newsweek Japan (in Japanese). 21 April 2020. Retrieved 5 June 2020.
- "日本が韓国の新型コロナウイルス対策から学べること──(4)軽症者の隔離・管理対策:「生活治療センター」". Newsweek Japan (in Japanese). 11 May 2020. Retrieved 5 June 2020.
- "韓国のコロナ対策を称える日本に欠ける視点". Newsweek Japan (in Japanese). 2 May 2020. Retrieved 5 June 2020.
- "韓国式大量検査は徴兵制の賜物…新型コロナが揺さぶる「自由」の価値" (in Japanese). FNNプライム. 14 April 2020. Retrieved 5 June 2020.
- "韓国における新型コロナウィルス防疫事情(韓国)" (in Japanese). 日本商工会議所. 10 May 2020. Retrieved 5 June 2020.
- "韓国製PCR検査キットが新型コロナから世界を救う日". Newsweek Japan (in Japanese). 14 April 2020. Retrieved 5 June 2020.
- "新型ウイルス"パンデミック" 医療崩壊を防ぐには". NHK (in Japanese). 9 April 2020. Retrieved 2 June 2020.
- "IT活用でコロナ追跡 韓国、感染者の経路公開". Mainichi Shimbun (in Japanese). 16 April 2020. Retrieved 5 June 2020.
- "コロナ対策で浮かび上がる「監視社会」韓国 個人情報をここまでさらしてよいのか". Tokyo Shimbun (in Japanese). 1 April 2020. Retrieved 5 June 2020.
- "新型コロナ:「感染追跡」デジタル監視とプライバシーの新しい日常" (in Japanese). Yahoo!ニュース. 26 March 2020. Retrieved 5 June 2020.
- "韓国、コロナ隔離者に監視腕輪 「人権侵害」の声" (in Japanese). The Nikkei. 17 April 2020. Retrieved 29 May 2020.
- "South Korea is watching quarantined citizens with a smartphone app". MIT Technology Review. 6 March 2020. Retrieved 5 June 2020.
- "Coronavirus privacy: Are South Korea's alerts too revealing?". BBC. 5 March 2020. Retrieved 5 June 2020.
- "台湾がコロナ「優等生」になった理由。閣僚に医師出身、デジタル化の一方で強まる監視". Business Insider (in Japanese). 1 May 2020. Retrieved 6 June 2020.
- "台湾の新型コロナ対策が「善戦」しているワケ". Wedge Infinity (in Japanese). 28 February 2020. Retrieved 6 June 2020.
- "台湾が新型コロナの感染拡大を抑制できている理由". Wedge Infinity (in Japanese). 28 February 2020. Retrieved 6 June 2020.
- "新型コロナ対応の「優等生」は「台湾・韓国・ドイツ」" (in Japanese). 日経ビジネス. 21 April 2020. Retrieved 6 June 2020.
- "COVID-19 Public Policies #2 ニューヨークはいかにして検査数を増やしたのか". Office of the City of Yokohama Representative to the Americas (in Japanese). 14 May 2020. Retrieved 2 June 2020.
- "Coronavirus New York: health officials provide limits on testing patients for COVID-19". EYEWITNESS NEWS. 21 March 2020. Retrieved 2 June 2020.
- "マスクも防護服も足りない! ニューヨークの病院で看護師が新型コロナウイルスに感染、死亡". Business Insider Japan (in Japanese). 27 March 2020. Retrieved 2 June 2020.
- "NY州感染者数、全米2位に 感染爆発で2週間封じ込め作戦へ". Yahoo!ニュース (in Japanese). 11 March 2020. Retrieved 2 June 2020.
- "Coronavirus clue? Most cases aboard U.S. aircraft carrier are symptom-free". Reuters. 16 April 2020.
- "Sailors on sidelined USS Theodore Roosevelt get virus for second time". NBC News. Retrieved 21 May 2020.
- "Special Report: Italy and South Korea virus outbreaks reveal disparity in deaths and tactics". Reuters. 13 March 2020. Retrieved 22 June 2020.
- "Bund sucht nicht mehr alle Corona-Infizierten". Der Bund (in German). ISSN 0774-6156. Retrieved 22 June 2020.
- "Want to know how many people have the coronavirus? Test randomly". The Conversation. 13 April 2020. Retrieved 7 May 2020.
- "M&E - Health Information System General Directorate - National Diseases Surveillance and Response". MoPH Data Warehouse - Dashboard. 31 May 2020.
- "9 qershor 2020/ Informacion i përditësuar për koronavirusin COVID-19". Ministria e Shëndetësisë dhe Mbrojtjes Sociale [Ministry of Health and Social Protection] (in Albanian). 9 June 2020.
- "COVID-19 dashboard". Africa CDC Dashboard. African Union. 20 June 2020.
- "Reporte diario matutino: situación de COVID-19 en Argentina". Argentina.gob.ar (in Spanish). 28 June 2020.
- Կորոնավիրուսային հիվանդություն (COVID-19). Հիվանդությունների վերահսկման և կանխարգելման ազգային կենտրոն [National Center for Disease Control and Prevention] (in Armenian). 24 May 2020.
- "Coronavirus (COVID-19) current situation and case numbers". Department of Health. 28 June 2020.
- "Coronavirus". Bundesministerium für Soziales, Gesundheit, Pflege und Konsumentenschutz [Federal Ministry of Social Affairs, Health, Care and Consumer Protection] (in German). 29 June 2020.
- "Azərbaycanda cari̇ vəzi̇yyət". KoronaVirus info (in Azerbaijani). Operational Headquarters under the Cabinet of Ministers. Retrieved 31 May 2020.
- فيروس الكورونا COVID-19. وزارة الصحة [Ministry of Health] (in Arabic). 22 June 2020.
- "Bangladesh Covid-19 Update". Institute of Epidemiology, Disease Control and Research. 26 June 2020.
- "COVID-19 Update: Two Women Test Positive Today". Barbados Government Information Service. 23 May 2020.
- У Беларусі на 24 чэрвеня выпісаныя 40 тыс. 136 пацыентаў. МІНІСТЭРСТВА АХОВЫ ЗДАРОЎЯ РЭСПУБЛІКІ БЕЛАРУСЬ [The Ministry of Health of the Republic of Belarus] (in Belarusian). 24 June 2020.
- "COVID-19 – Epidemiologisch bulletin van 23 mei 2020" (PDF). Sciensano (in Dutch). 23 May 2020.
- "COVID-19 in Bhutan". Ministry of Health. 5 June 2020.
- "Ministerio de Salud reporta 1036 contagios nuevos de coronavirus y el total sube a 23512". Ministerio de Salud [Ministry of Health] (in Spanish). 20 June 2020.
- "Službene informacije o koronavirusu u BiH". Ministarstvo civilnih poslova Bosne i Hercegovine [Ministry of Civil Affairs of Bosnia and Herzegovina] (in Bosnian). 24 June 2020.
- "Painel de Leitos e Insumos". Ministério da Saúde [Ministry of Health] (in Portuguese). 4 June 2020.
- "Coronavírus Brasil". Ministério da Saúde [Ministry of Health] (in Portuguese). 4 June 2020.
- COVID-19: Единен информационен портал. COVID-19: Единен информационен портал [COVID-19: United information portal] (in Bulgarian). 24 May 2020.
- "Ministère de la Santé". Ministère de la Santé [Ministry of Health] (in French). 24 May 2020.
- "www.facebook.com/1444809365833949/photos/a.1590964164551801/2535911953390346". Ministère de la Santé - Burkina Faso (Facebook) [Ministry of Health - Burkina Faso (Facebook)] (in French). 22 May 2020.
- "Africa CDC Dashboard". Africa CDC.
- "Coronavirus disease (COVID-19): Outbreak update". Government of Canada. Retrieved 26 June 2020.
- "Cifras Oficiales: COVID-19". Gob.cl (in Spanish). 25 June 2020.
- "Officials: Testing key to virus control". National Health Commission of the People's Republic of China. 26 June 2020.
- Jiang, Steven; Deng, Shawn; Yee, Isaac (24 June 2020). "China says it has conducted more than 90 million tests for coronavirus". CNN.
- "June 23: Daily briefing on novel coronavirus cases in China". National Health Commission of the People's Republic of China. 23 June 2020.
- 国务院联防联控机制就做好重点场所重点单位重点人群疫情防控工作情况举行发布会. 中国网 [China Net] (in Chinese). 15 April 2020.
- "Coronavirus (COVID - 2019) en Colombia". Instituto Nacional de Salud [National Institute of Health] (in Spanish). 21 June 2020.
- "2213 casos confirmados por COVID-19". Ministerio de Salud, Costa Rica [Ministry of Health, Costa Rica] (in Spanish). 21 June 2020.
- "Jedan novooboljeli u posljednja 24 sata". Koronavirus.hr (in Croatian). 9 June 2020.
- "Covid19CubaData". Covid19CubaData (in Spanish). 21 June 2020.
- Η εξάπλωση του COVID-19 στην Κύπρο. Πανεπιστήμιο Κύπρου [University of Cyprus] (in Greek). 23 May 2020.
- "Přehled situace v ČR: COVID-19". Ministerstvo zdravotnictví České republiky [The Ministry of Health of the Czech Republic] (in Czech). 26 June 2020.
- "Overvågning af COVID-19". Statens Serum Institut [State Serum Institute] (in Danish). 22 June 2020.
- "COVID-19 dashboard". Africa CDC Dashboard. African Union. 2 June 2020.
- "Informes de Situación e Infografias – COVID 19" (PDF). Ministerio de Salud Pública [Ministry of Public Health] (in Spanish). 20 June 2020.
- "Situación nacional COVID-19". Gobierno de El Salvador [Government of El Salvador] (in Spanish). 21 June 2020.
- "Koroonakaart". Koroonakaart. 8 June 2020.
- "Notification Note on COVID-19 Situational Update" (PDF). የኢትዮጵያ የሕብረተሰብ ጤና ኢንሰቲትዩት [Ethiopian Public Health Institute]. 23 June 2020. p. 2.
- "Confirmed coronavirus cases (COVID-19) in Finland". Terveyden ja hyvinvoinnin laitos (ArcGIS) [National Institute for Health and Welfare (ArcGIS)]. 20 June 2020.
- "COVID-19 : Point épidémiologique hebdomadaire du 7 mai 2020" (PDF). Santé publique France [Public Health France] (in French). 7 May 2020.
- "COVID-19 : Point épidémiologique hebdomadaire du 29 mai 2020" (PDF). Santé publique France [Public Health France] (in French). 29 May 2020.
- "Nombre de pesonnes testées - tous âges". France official. Retrieved 4 June 2020.
- "Coronavirus Disease 2019 (COVID-19) Daily Situation Report" (PDF). Robert Koch-Institut [Robert Koch Institute]. 10 June 2020.
- "Disease Surveillance Dept_GHS". The Official Twitter account of Disease Surveillance Department, Ghana Health Service. 7 May 2020.
- Ημερήσιες Εκθέσεις COVID-19. Εθνικός Οργανισμός Δημόσιας Υγείας [National Public Health Organization] (in Greek). 10 May 2020.
- "Grenada's current #COVID19 statistics - June 18, 2020 at 10:12 am". Ministry of Health Grenada (Facebook). 18 June 2020.
- "Tájékoztató oldal a koronavírusról". Tájékoztató oldal a koronavírusról [Coronavirus Information Page] (in Hungarian). Cabinet Office of the Prime Minister. 8 June 2020.
- "COVID-19 in Iceland – Statistics". Covid.is. 22 June 2020.
- "SARS-CoV-2 (COVID-19) Testing: Status Update". Indian Council of Medical Research. Retrieved 17 June 2020.
- "Ministry of Health and Family Welfare". Ministry of Health and Family Welfare. Retrieved 17 June 2020.
- "COVID-19". Kementerian Kesehatan Indonesia [Indonesian Health Ministry] (in Indonesian). 28 June 2020.
- "Health Ministry Says COVID-19 Death Toll Rises to 7,300". Government of the Islamic Republic of Iran. 22 May 2020.
- "www.facebook.com/156490947738645/posts/2927020600685652/". وزارة الصحة العراقية (Facebook) [Iraqi Ministry of Health (Facebook)] (in Arabic). 7 May 2020.
- "Ireland's COVID-19 Data Hub". gov.ie. 27 June 2020.
- קורונה - לוח בקרה. נגיף הקורונה [Coronavirus] (in Hebrew). Ministry of Health. 24 June 2020.
- "Aggiornamento 24/06/2020 ore 17.00" (PDF). Dipartimento della Protezione Civile (GitHub) [Civil Protection Department (GitHub)] (in Italian). 24 June 2020.
- "Jamaica's COVID-19 recoveries reach 100". Ministry of Health & Wellness. 12 May 2020.
- 新型コロナウイルス感染症の現在の状況について(令和2年5月24日版). 厚生労働省 [The Ministry of Health, Labour and Welfare] (in Japanese). 19 June 2020.
- Национальный центр общественного здравоохранения Министерства здравоохранения Республики Казахстан. Национальный центр общественного здравоохранения Министерства здравоохранения Республики Казахстан [National Center of Public Health of the Ministry of Healthcare of the Republic of Kazakhstan] (in Russian). 31 May 2020.
- Cheruiyot, Kevin (10 May 2020). "Kenya confirms 23 new cases, totaling to 672". The Star (Kenya).
- "Njëzet e dy të shëruar dhe katër raste me COVID-19!". Instituti Kombëtar i Shëndetësisë Publike të Kosovës [National Institute of Public Health of Kosova] (in Albanian). Retrieved 9 June 2020.
- Эпидситуация по COVID-19 в Кыргызстане на 5 мая. Министерство здравоохранения Кыргызской Республики [Ministry of Health of the Kyrgyz Republic] (in Russian). 5 May 2020.
- "Covid-19 infekcijas izplatība Latvijā 29.01.2020.-16.05.2020". Slimību profilakses un kontroles centrs (ArcGIS) [Center for Disease Prevention and Control (ArcGIS)] (in Latvian). 8 June 2020.
- آخر اﻹحصاءات. فيروس كورونا: COVID-19 [Coronavirus: COVID-19] (in Arabic). Ministry of Information. 12 May 2020.
- "Koronavirusas (COVID-19)". Lietuvos Respublikos sveikatos apsaugos ministerija [Ministry of Health of the Republic of Lithuania] (in Lithuanian). 24 June 2020.
- "Coronavirus". Coronavirus. 29 June 2020.
- "www.facebook.com/malawimoh/photos/pcb.2943775645677931/2943775575677938/?type=3&theater". Ministry Of Health - Malawi (Facebook). 21 June 2020.
- "KKMPutrajaya on Twitter". The Official twitter for Ministry Of Health, Malaysia (in Malay). 16 June 2020.
- "www.facebook.com/MinistryOfHealthMV/videos/242937120374212/". Ministry of Health (Facebook). 22 May 2020.
- "COVID-19 Malta". Times of Malta (ArcGIS). 26 May 2020.
- "Covid-19 : Communiqués". Republic of Mauritius. 2 June 2020.
- "Covid-19 México". Gobierno de México [Government of Mexico] (in Spanish). 28 June 2020.
- "Uživo: COVID-19". Institut za javno zdravlje Crne Gore [Institute of Public Health of Montenegro] (in Montenegrin). 5 May 2020.
- مرض فيروس كورونا المستجد: الرصد الصحي بالمغرب. البوابة الرسمية لفيروس كورونا بالمغرب [The official portal of coronavirus in Morocco] (in Arabic). 2 June 2020.
- "Ministério da Saúde". Ministério da Saúde [Ministry of Health] (in Portuguese). 10 May 2020.
- "Ministry of Health and Sports". Ministry of Health and Sports (in Burmese). 31 May 2020.
- "Corona Info". Corona Info (in Nepali). Ministry of Health and Population. 24 June 2020.
- "Epidemiologische situatie COVID-19 in Nederland" (PDF). Rijksinstituut voor Volksgezondheid en Milieu [National Institute for Public Health and the Environment] (in Dutch). 20 May 2020.
- "COVID-19 - current cases". Ministry of Health. 20 June 2020.
- "Coronavirus COVID-19 Microsite". Coronavirus COVID-19 Microsite. Nigeria Centre for Disease Control. 7 June 2020.
- Kim, Jeongmin (22 April 2020). "740 people in North Korea tested for COVID-19, still no confirmed cases: WHO". NK News. Retrieved 7 May 2020.
- ДЕНЕСКА СЕ НОВИ СЛУЧАИ НА КОВИД ПОТВРДЕНИ 19 – ВКУПНАТА БРОЈКА НА ДИЈАГНОСТИЦИРАНИ 2315, ОЗДРАВЕНИ СЕ 17 ПАЦИЕНТИ – ПОЧИНАТИ СЕ 7 ЛИЦА. Министерство за здравство [Ministry of Health] (in Macedonian). 1 June 2020.
- "COVID-19 Genel Durum". Kuzey Kıbrıs Türk Cumhuriyeti Sağlık Bakanlığı [Turkish Republic of Northern Cyprus Ministry of Health] (in Turkish). 30 May 2020.
- "Dags- og ukerapporter om koronavirussykdom (covid-19)". Folkehelseinstituttet [Norwegian Institute of Public Health] (in Norwegian). 22 May 2020.
- "Oman tests more than 175,000 people for coronavirus". The Arabian Stories. 26 June 2020.
- "Pakistan Cases Details". COVID-19 Health Advisory Platform. Ministry of National Health Services Regulations and Coordination. Retrieved 28 June 2020.
- فايروس كورونا (COVID-19) في فلسطين. فايروس كورونا (COVID-19) في فلسطين [Coronavirus (COVID-19) in Palestine] (in Arabic). Retrieved 30 June 2020.
- "Casos de Coronavirus COVID-19 en Panamá". Ministerio de Salud [Ministry of Health] (in Spanish). 9 June 2020.
- "Reportes - COVID19". Ministerio de Salud Pública y Bienestar Social [Ministry of Public Health and Social Welfare] (in Spanish). 19 June 2020.
- "Sala Situacional: COVID-19 Perú". Covid-19 en el Perú [Covid-19 in Peru] (in Spanish). 29 June 2020.
- "COVID-19 Tracker". Department of Health. 30 June 2020.
- "twitter.com/MZ_GOV_PL/status/1267418113487917056". Ministerstwo Zdrowia (Twitter) [Ministry of Health (Twitter)] (in Polish). 1 June 2020.
- "Ponto de Situação Atual em Portugal". COVID-19 (in Portuguese). Ministry of Health. 17 May 2020.
- "COVID19 Home". Ministry of Public Health. 26 June 2020.
- "Buletin informativ 28.05.2020". Ministerul Sănătăţii [Ministry of Health] (in Romanian). 28 May 2020.
- Информационный бюллетень о ситуации и принимаемых мерах по недопущению распространения заболеваний, вызванных новым коронавирусом. Федеральная служба по надзору в сфере защиты прав потребителей и благополучия человека (Роспотребнадзор) [Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)] (in Russian). 29 June 2020.
- О подтвержденных случаях новой коронавирусной инфекции COVID-2019 в России. Федеральная служба по надзору в сфере защиты прав потребителей и благополучия человека (Роспотребнадзор) [Federal Service for Surveillance on Consumer Rights Protection and Human Wellbeing (Rospotrebnadzor)] (in Russian). 29 June 2020.
- "twitter.com/RwandaHealth". Ministry of Health (Twitter). 21 June 2020.
- "SAINT LUCIA'S COVID-19 DASHBOARD". Ministry of Health and Wellness. 25 May 2020.
- "COVID 19 Dashboard: Saudi Arabia". Ministry of Health. Retrieved 9 June 2020.
- "github.com/senegalouvert/COVID-19/blob/master/data/confirmes.csv". Senegal Ouvert (GitHub) [Senegal Open (GitHub)] (in French). 11 May 2020.
- Информација о новом корона вирусу на дан 28. мај 2020. године у 15 часова. Министарство здравља [Ministry of Health] (in Serbian). 28 May 2020.
- "Updates on COVID-19 (Coronavirus Disease 2019) Local Situation". Ministry of Health. 22 June 2020.
- "22 June 2020 Daily Report on COVID-19" (PDF). Ministry of Health. 22 June 2020.
- "Coronavirus (COVID-19) in the Slovak Republic". korona.gov.sk. Office of the Deputy Prime Minister of the Slovak Republic for Investments and Informatization. 29 June 2020.
- "Dnevno spremljanje okužb s SARS-CoV-2 (COVID-19)". Nacionalni inštitut za javno zdravje [National Institute of Public Health] (in Slovenian). 7 June 2020.
- "COVID-19 South African coronavirus news and information". South African Government. 6 June 2020.
- 코로나바이러스감염증-19(COVID-19). 코로나바이러스감염증-19(COVID-19) [Coronavirus infection-19 (COVID-19)] (in Korean). Ministry of Health and Welfare. 28 June 2020.
- "Pruebas diagnósticas" (PDF). Ministerio de Sanidad, Consumo y Bienestar Social [Ministry of Health, Consumption and Social Welfare] (in Spanish). 23 May 2020.
- "Actualización no 106. Enfermedad por el coronavirus (COVID-19)" (PDF). Ministerio de Sanidad, Consumo y Bienestar Social [Ministry of Health, Consumption and Social Welfare] (in Spanish). 15 May 2020.
- "COVID-19 Situation Report". Health Promotion Bureau, Sri Lanka. 17 June 2020.
- "Veckorapport om covid-19, vecka 24" (PDF). Folkhälsomyndigheten [Public Health Agency of Sweden] (in Swedish). 17 June 2020. p. 5.
- "Coronavirus Krankheit 2019 (COVID-19): Situationsbericht zur epidemiologischen Lage in der Schweiz und im Fürstentum Liechtenstein" (PDF). Bundesamt für Gesundheit [The Federal Office of Public Health] (in German). 16 June 2020. p. 1.
- "Taiwan Centers for Disease Control". Taiwan Centers for Disease Control. 29 June 2020.
- "The Coronavirus Disease 2019 Situation" (PDF). Department of Disease Control. 7 May 2020.
- "COVID-19 (Novel Coronavirus)". Ministry of Health. 29 June 2020.
- "Tableau de Bord". Covid 19 Tunisia (in French). Ministry of Health. 9 May 2020.
- "Korona Tablosu". Korona Tablosu [Corona Table] (in Turkish). Ministry of Health. 29 June 2020.
- "MoH Uganda: COVID-19 Information Portal". MoH Uganda: COVID-19 Information Portal. 21 June 2020.
- "Coronavirus in Ukraine". Coronavirus in Ukraine. Cabinet of Ministers of Ukraine. 12 May 2020.
-
- "UAE Government: Over two million COVID-19 tests conducted, 822 new cases identified". Ministry of Health & Prevention. 25 May 2020.
- "MoHAP conducts over 28,000 additional COVID-19 tests, announces 779 new cases, 325 recovers and 5 deaths". Ministry of Health & Prevention. 26 May 2020.
- "COVID-19 recoveries rise to 16,371; 883 new cases identified". Ministry of Health & Prevention. 27 May 2020.
- "MoHAP announces 38,000 additional COVID-19 tests as part of intensified screening, 563 new cases, 314 recoveries and three deaths". Ministry of Health & Prevention. 28 May 2020.
- "MoHAP announces over 36,000 additional COVID-19 tests as part of intensified screening, 638 new cases, 412 recoveries and two deaths". Ministry of Health & Prevention. 29 May 2020.
- "UAE Government: COVID-19 recoveries rise to 17,546; new 726 cases identified". Ministry of Health & Prevention. 30 May 2020.
- "UAE: Rise in COVID-19 recoveries to 17,932, 661 new cases identified and 2 deaths". Ministry of Health & Prevention. 31 May 2020.
- "UAE Government: COVID-19 recoveries rise to over 18,000; 635 new cases identified". Ministry of Health & Prevention. 1 June 2020.
- "MoHAP conducts over 35,000 additional COVID-19 tests, announces 596 new cases". Ministry of Health & Prevention. 2 June 2020.
- "UAE Government: COVID-19 recoveries rise to over 19,000; 571 new cases identified". Ministry of Health & Prevention. 3 June 2020.
- "MoHAP conducts over 54,000 additional COVID-19 tests, announces 659 new cases, 419 recoveries, 3 deaths". Ministry of Health & Prevention. 4 June 2020.
- "MoHAP conducts over 44,000 additional COVID-19 tests, announces 624 new cases, 765 recoveries, one death". Ministry of Health & Prevention. 5 June 2020.
- "MoHAP conducts over 44,000 additional COVID-19 tests, announces 540 new cases, 745 recoveries, one death". Ministry of Health & Prevention. 7 June 2020.
- "MoHAP conducts over 32,000 additional COVID-19 tests, announces 568 new cases, 469 recoveries, five deaths". Ministry of Health & Prevention. 8 June 2020.
- "MoHAP conducts over 37,000 additional COVID-19 tests, announces 528 new cases". Ministry of Health & Prevention. 9 June 2020.
- "UAE conducts over 47,000 additional COVID-19 tests; announces 603 new cases, 1,277 recoveries, one death". Ministry of Health & Prevention. 10 June 2020.
- "MoHAP conducts over 45,000 additional COVID-19 tests, announces 479 new cases". Ministry of Health & Prevention. 11 June 2020.
- "MoHAP conducts over 44,000 additional COVID-19 tests, announces 513 new cases, 712 recoveries, one death". Ministry of Health & Prevention. 12 June 2020.
- "MoHAP conducts over 40,000 additional COVID-19 tests, announces 491 new cases, 815 recoveries, one death". Ministry of Health & Prevention. 13 June 2020.
- "MoHAP conducts over 43,000 additional COVID-19 tests, announces 304 new cases, 701 recoveries, one death". Ministry of Health & Prevention. 14 June 2020.
- "MOHAP conducts over 27,000 additional COVID-19 tests, announces 342 new cases, 667 recoveries, two deaths". Ministry of Health & Prevention. 15 June 2020.
- "MoHAP conducts over 38,000 additional COVID-19 tests, announces 346 new cases, 732 recoveries and 2 death". Ministry of Health & Prevention. 16 June 2020.
- "MoHAP conducts over 40,000 additional COVID-19 tests, announces 388 new cases, 704 recoveries, three deaths". Ministry of Health & Prevention. 18 June 2020.
- "MoHAP conducts over 38,000 additional COVID-19 tests, announces 393 new cases, 755 recoveries, 2 death". Ministry of Health & Prevention. 19 June 2020.
- "MoHAP conducts over 34,000 additional COVID-19 tests, announces 388 new cases, 758 recoveries and 1 death". Ministry of Health & Prevention. 20 June 2020.
- "MoHAP conducts over 48,000 additional COVID-19 tests, announces 392 new cases, 661 recoveries and 1 death". Ministry of Health & Prevention. 21 June 2020.
- "MoHAP announces 380 new COVID-19 cases, 657 recoveries and 2 deaths". Ministry of Health & Prevention. 23 June 2020.
- "Number of coronavirus (COVID-19) cases and risk in the UK". GOV.UK. 29 June 2020.
- covidtracking. "US Historical Data". covidtracking. Retrieved 6 June 2020.
- "Covid Tracking US Daily". covidtracking.com. 21 June 2020.
- "Informe de situación en relación al coronavirus COVID-19 en Uruguay (19 06 2020)". Sistema Nacional de Emergencias [National Emergency System] (in Spanish). 19 June 2020.
- "t.me/koronavirusinfouz/3470". koronavirusinfouz (Telegram) (in Uzbek). 7 May 2020.
- "Venezuela suma 198 nuevos casos de COVID-19 y contabiliza 3 mil 790 infecciones". Ministerio del Poder Popular para la Comunicación y la Información [Ministry of Popular Power for Communication and Information] (in Spanish). 20 June 2020.
- "COVID-19: Cập nhật mới nhất, liên tục". Sức khỏe và Ðời sống [Health and Life] (in Vietnamese). Ministry of Health. 15 June 2020.
- "Zimbabwe COVID-19 Cases". Zimbabwe COVID-19 Cases. Retrieved 21 June 2020.
External links
| Wikimedia Commons has media related to COVID-19 testing. |
| Wikiquote has quotations related to: COVID-19 testing |
- "COVID-19 diagnostic tech tableau". BioCentury. Retrieved 22 June 2020.
- Data on COVID-19 testing at Our World in Data (31 March 2020)
- COVID-19 Testing (at least) – now Free for all? (CDC; US Congress; CSPAN video/6:00; March 12, 2020)
- Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR; NCBI-PMC, DOI (23 January 2020)
- "EUA Authorized Serology Test Performance". U.S. Food and Drug Administration (FDA). 4 June 2020.
- "Global Progress on COVID-19 Serology-Based Testing". Johns Hopkins Center for Health Security. 5 June 2020. Retrieved 9 June 2020.
- "EUA Authorized Serology Test Performance". FDA. 22 May 2020. Retrieved 29 May 2020.
- Health, Center for Devices and Radiological (15 June 2020). "EUA Authorized Serology Test Performance". FDA.