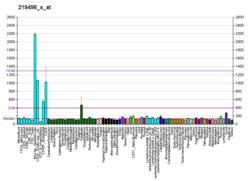
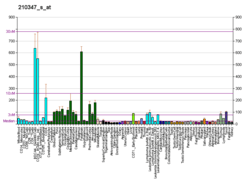
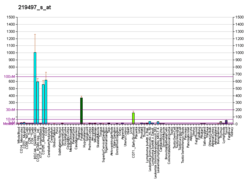
BCL11A
B-cell lymphoma/leukemia 11A is a protein that in humans is encoded by the BCL11A gene.[5][6][7]
Function
The BCL11A gene encodes for a regulatory C2H2 type zinc-finger protein, that can bind to the DNA. Five alternatively spliced transcript variants of this gene, which encode distinct isoforms, have been reported.[7] The protein associates with the SWI/SNF complex, that regulates gene expression via chromatin remodelling.[8]
BCL11A is highly expressed in several hematopoietic lineages, and plays a role in the switch from γ- to β-globin expression during the fetal to adult erythropoiesis transition.[9]
Furthermore, BCL11A is expressed in the brain, where it forms a protein complex with CASK to regulate axon outgrowth and branching.[10] In the neocortex, BCL11A binds to the TBR1 regulatory region and inhibits the expression of TBR1.[11]
Clinical significance
The corresponding Bcl11a mouse gene is a common site of retroviral integration in myeloid leukemia, and may function as a leukemia disease gene, in part, through its interaction with BCL6. During hematopoietic cell differentiation, this gene is down-regulated. It is possibly involved in lymphoma pathogenesis since translocations associated with B-cell malignancies also deregulates its expression. In addition, BCL11A has been found to play a role in the suppression of fetal hemoglobin production. Therapeutic strategies aimed at increasing fetal globin production in diseases such as beta thalassemia and sickle cell anemia by inhibiting BCL11A are currently being explored.
Furthermore, heterozygous de novo mutations in BCL11A have been identified in an intellectual disability disorder, accompanied with global developmental delay and autism spectrum disorder.[12] These mutations disrupt BCL11A homodimerization and transcriptional regulation.
Interactions
BCL11A has been shown to interact with a number of proteins. BCL11A was initially discovered as a COUP-TFI interacting protein.[13] In the nucleus, BCL11A forms paraspeckles that co-localize with NONO.[12] In neurons, BCL11A interacts with CASK to regulate target genes.[10] Furthermore, BCL11A interacts with the neuron-specific protein TBR1, which is also implicated in intellectual disability and autism spectrum disorder.[14]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000119866 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000000861 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Satterwhite E, Sonoki T, Willis TG, Harder L, Nowak R, Arriola EL, Liu H, Price HP, Gesk S, Steinemann D, Schlegelberger B, Oscier DG, Siebert R, Tucker PW, Dyer MJ (December 2001). "The BCL11 gene family: involvement of BCL11A in lymphoid malignancies". Blood. 98 (12): 3413–20. doi:10.1182/blood.V98.12.3413. PMID 11719382.
- ↑ Uda M, Galanello R, Sanna S, Lettre G, Sankaran VG, Chen W, et al. (February 2008). "Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia". Proceedings of the National Academy of Sciences of the United States of America. 105 (5): 1620–5. doi:10.1073/pnas.0711566105. PMC 2234194. PMID 18245381.
- 1 2 "Entrez Gene: BCL11A B-cell CLL/lymphoma 11A (zinc finger protein)".
- ↑ Kadoch C, Hargreaves DC, Hodges C, Elias L, Ho L, Ranish J, Crabtree GR (June 2013). "Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy". Nature Genetics. 45 (6): 592–601. doi:10.1038/ng.2628. PMC 3667980. PMID 23644491.
- ↑ Smith EC, Luc S, Croney DM, Woodworth MB, Greig LC, Fujiwara Y, Nguyen M, Sher F, Macklis JD, Bauer DE, Orkin SH (November 2016). "Bcl11a erythroid enhancer". Blood. 128 (19): 2338–2342. doi:10.1182/blood-2016-08-736249. PMC 5106112. PMID 27707736.
- 1 2 Kuo TY, Hong CJ, Chien HL, Hsueh YP (August 2010). "X-linked mental retardation gene CASK interacts with Bcl11A/CTIP1 and regulates axon branching and outgrowth". Journal of Neuroscience Research (in German). 88 (11): 2364–73. doi:10.1002/jnr.22407. PMID 20623620.
- ↑ Cánovas J, Berndt FA, Sepúlveda H, Aguilar R, Veloso FA, Montecino M, Oliva C, Maass JC, Sierralta J, Kukuljan M (May 2015). "The Specification of Cortical Subcerebral Projection Neurons Depends on the Direct Repression of TBR1 by CTIP1/BCL11a". The Journal of Neuroscience. 35 (19): 7552–64. doi:10.1523/JNEUROSCI.0169-15.2015. PMID 25972180.
- 1 2 Dias C, Estruch SB, Graham SA, McRae J, Sawiak SJ, Hurst JA, Joss SK, Holder SE, Morton JE, Turner C, Thevenon J, Mellul K, Sánchez-Andrade G, Ibarra-Soria X, Deriziotis P, Santos RF, Lee SC, Faivre L, Kleefstra T, Liu P, Hurles ME, Fisher SE, Logan DW (August 2016). "BCL11A Haploinsufficiency Causes an Intellectual Disability Syndrome and Dysregulates Transcription". American Journal of Human Genetics. 99 (2): 253–74. doi:10.1016/j.ajhg.2016.05.030. PMC 4974071. PMID 27453576.
- ↑ Avram D, Fields A, Senawong T, Topark-Ngarm A, Leid M (December 2002). "COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein". The Biochemical Journal. 368 (Pt 2): 555–63. doi:10.1042/bj20020496. PMC 1223006. PMID 12196208.
- ↑ den Hoed J, Sollis E, Venselaar H, Estruch SB, Deriziotis P, Fisher SE (September 2018). "Functional characterization of TBR1 variants in neurodevelopmental disorder". Scientific Reports. 8 (1): 14279. doi:10.1038/s41598-018-32053-6. PMC 6155134. PMID 30250039.
Further reading
- "Toward a complete human genome sequence". Genome Research. 8 (11): 1097–108. November 1998. doi:10.1101/gr.8.11.1097. PMID 9847074.
- Avram D, Fields A, Pretty On Top K, Nevrivy DJ, Ishmael JE, Leid M (April 2000). "Isolation of a novel family of C(2)H(2) zinc finger proteins implicated in transcriptional repression mediated by chicken ovalbumin upstream promoter transcription factor (COUP-TF) orphan nuclear receptors". The Journal of Biological Chemistry. 275 (14): 10315–22. doi:10.1074/jbc.275.14.10315. PMC 2819356. PMID 10744719.
- Nakamura T, Yamazaki Y, Saiki Y, Moriyama M, Largaespada DA, Jenkins NA, Copeland NG (May 2000). "Evi9 encodes a novel zinc finger protein that physically interacts with BCL6, a known human B-cell proto-oncogene product". Molecular and Cellular Biology. 20 (9): 3178–86. doi:10.1128/MCB.20.9.3178-3186.2000. PMC 85612. PMID 10757802.
- Saiki Y, Yamazaki Y, Yoshida M, Katoh O, Nakamura T (December 2000). "Human EVI9, a homologue of the mouse myeloid leukemia gene, is expressed in the hematopoietic progenitors and down-regulated during myeloid differentiation of HL60 cells". Genomics. 70 (3): 387–91. doi:10.1006/geno.2000.6385. PMID 11161790.
- Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O (April 2001). "Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro". DNA Research. 8 (2): 85–95. doi:10.1093/dnares/8.2.85. PMID 11347906.
- Martín-Subero JI, Gesk S, Harder L, Sonoki T, Tucker PW, Schlegelberger B, Grote W, Novo FJ, Calasanz MJ, Hansmann ML, Dyer MJ, Siebert R (February 2002). "Recurrent involvement of the REL and BCL11A loci in classical Hodgkin lymphoma". Blood. 99 (4): 1474–7. doi:10.1182/blood.V99.4.1474. PMID 11830502.
- Küppers R, Sonoki T, Satterwhite E, Gesk S, Harder L, Oscier DG, Tucker PW, Dyer MJ, Siebert R (May 2002). "Lack of somatic hypermutation of IG V(H) genes in lymphoid malignancies with t(2;14)(p13;q32) translocation involving the BCL11A gene". Leukemia. 16 (5): 937–9. doi:10.1038/sj.leu.2402480. PMID 11986957.
- Senawong T, Peterson VJ, Leid M (February 2005). "BCL11A-dependent recruitment of SIRT1 to a promoter template in mammalian cells results in histone deacetylation and transcriptional repression". Archives of Biochemistry and Biophysics. 434 (2): 316–25. doi:10.1016/j.abb.2004.10.028. PMC 2819353. PMID 15639232.
- Liu H, Ippolito GC, Wall JK, Niu T, Probst L, Lee BS, Pulford K, Banham AH, Stockwin L, Shaffer AL, Staudt LM, Das C, Dyer MJ, Tucker PW (May 2006). "Functional studies of BCL11A: characterization of the conserved BCL11A-XL splice variant and its interaction with BCL6 in nuclear paraspeckles of germinal center B cells". Molecular Cancer. 5: 18. doi:10.1186/1476-4598-5-18. PMC 1526750. PMID 16704730.
- Weniger MA, Pulford K, Gesk S, Ehrlich S, Banham AH, Lyne L, Martin-Subero JI, Siebert R, Dyer MJ, Möller P, Barth TF (October 2006). "Gains of the proto-oncogene BCL11A and nuclear accumulation of BCL11A(XL) protein are frequent in primary mediastinal B-cell lymphoma". Leukemia. 20 (10): 1880–2. doi:10.1038/sj.leu.2404324. PMID 16871282.
- Szafranski K, Schindler S, Taudien S, Hiller M, Huse K, Jahn N, Schreiber S, Backofen R, Platzer M (2007). "Violating the splicing rules: TG dinucleotides function as alternative 3' splice sites in U2-dependent introns". Genome Biology. 8 (8): R154. doi:10.1186/gb-2007-8-8-r154. PMC 2374985. PMID 17672918.
External links
- BCL11A+protein,+human at the US National Library of Medicine Medical Subject Headings (MeSH)
- FactorBook BCL11A
- Human BCL11A genome location and BCL11A gene details page in the UCSC Genome Browser.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.