Normethandrone
 | |
| Clinical data | |
|---|---|
| Trade names | Metalutin, others |
| Synonyms | Normetandrone; Methylestrenolone; Methyloestrenolone; Methylnortestosterone; Normethyltestosterone; Normethandrolone; Normethisterone; Methylnandrolone; NMT; 17α-Methyl-19-nortestosterone; 17α-Methylestr-4-en-17β-ol-3-one; P-6051; RU-598; NSC-10039 |
| Routes of administration | By mouth |
| Drug class | Progestin; Progestogen; Androgen; Anabolic steroid |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard |
100.007.440 |
| Chemical and physical data | |
| Formula | C19H28O2 |
| Molar mass | 288.42 g/mol |
| 3D model (JSmol) | |
| |
| |
Normethandrone, also known as methylestrenolone or methylnortestosterone and sold under the brand name Metalutin among others, is a progestin and androgen/anabolic steroid (AAS) medication which is used in combination with an estrogen in the treatment of amenorrhea and menopausal symptoms in women.[1][2][3][4] It is taken by mouth.[5]
Side effects of normethandrone include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[6] It can also cause liver damage.[7] Normethandrone is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[5] It is also a synthetic AAS and hence is an agonist of the androgen receptor, the biological target of androgens like testosterone and dihydrotestosterone (DHT).[4][8] It has some estrogenic activity as well and no other important hormonal activity.[9][1][3]
Normethandrone was introduced for medical use by 1957.[10] It is available only in a few countries, including Brazil, Indonesia, and Venezuela, and is available only in combination with methylestradiol or estradiol valerate.[2][1]
Medical uses
Normethandrone is used in combination with an estrogen, either methylestradiol or estradiol valerate, in the treatment of amenorrhea and menopausal symptoms in women.[1][2][11] It has also been used to treat dysmenorrhea in women.[12] Normethandrone has been used successfully to inhibit libido in men with sexual deviance.[13] Although normethandrone can be classified as an AAS and has strong such effects at sufficiently high doses, it is not typically used as such and is instead used medically only as a progestin.[3][1][4] This is because it is so highly progestogenic in comparison.[4]
Available forms
Normethandrone is marketed in combination with methylestradiol in the form of oral tablets containing 5 mg normethandrone and 0.3 mg methylestradiol.[11][14]
Side effects
Normethandrone has been associated with symptoms of masculinization and hepatotoxicity.[6][7]
Pharmacology
Pharmacodynamics
Normethandrone shows high progestogenic activity.[5] With sublingual administration in women, it has at least 150 times the potency of sublingual progesterone and 50 times the potency of sublingual ethisterone.[5] It also has 10 times the potency of injected progesterone via this route.[5] In addition to its progestogenic activity, normethandrone has anabolic and androgenic activity and can produce effects associated with this activity.[1][4] It has a high ratio of anabolic to androgenic activity.[15] The anabolic potency of normethandrone is similar to that of norethandrolone and is much greater than that of nandrolone or metandienone.[8] It is also greater than that of ethylestrenol.[8] In addition to its progestogenic and androgenic/anabolic activity, normethandrone has estrogenic activity via aromatization into methylestradiol.[3]
Analogously to nandrolone and norethandrolone, 5α-dihydronormethandrone, the 5α-reduced metabolite of normethandrone, shows reduced affinity for the androgen receptor relative to normethandrone.[16][17] Its affinity for the androgen receptor is specifically about 33 to 60% of that of normethandrone.[16]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | |
|---|---|---|---|---|---|---|---|---|
| Normethandrone | 75–125 | 125–150 | <1 | 1–5 | <1 | ? | ? | |
| 5α-Dihydronormethandrone | 15–25 | 50–75 | ? | <1 | ? | ? | ? | |
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. | ||||||||
Pharmacokinetics
Normethandrone is metabolized by aromatase into methylestradiol in small quantities, similarly to methyltestosterone and metandienone.[3][20][21] The metabolites of normethandrone have not been well-studied, but 5α-dihydronormethandrone is a likely metabolite formed by 5α-reductase.[22][23]
The pharmacokinetics of normethandrone have been reviewed.[24]
Chemistry
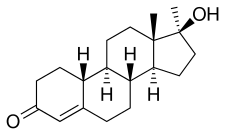
Normethandrone, also known as 17α-methyl-19-nortestosterone or as 17α-methylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a 17α-alkylated derivative of nandrolone (19-nortestosterone; 19-NT). It is specifically the 17α-methyl derivative of nandrolone as well as the 17α-methyl variant of norethandrolone (17α-ethyl-19-NT) and norethisterone (17α-ethynyl-19-NT).[25]
Synthesis
Chemical syntheses of normethandrone have been published.[24]
History
Normethandrone has been marketed for medical use since 1957.[10] The combination of normethandrone and methylestradiol was introduced by at least 1966.[14]
Society and culture
Generic names
Normethandrone has not been assigned an INN or other formal name designations.[25][26][2] It is also known as methylestrenolone, methylnortestosterone, normethandrolone, and normethisterone.[25][26][2]
Brand names
Brand names of normethandrone include Batynid, Ginecosid, Ginecoside, Gynomin, Lutenin, Matronal, Mediol, Metalutin, Methalutin, Orgasteron, Orosteron, and Renodiol.[25][26][2][1][27][11]
Availability
Normethandrone is marketed in Brazil, Indonesia, and Venezuela in combination with methylestradiol or estradiol valerate.[2][1]
References
- 1 2 3 4 5 6 7 8 https://www.medicinescomplete.com/about/
- 1 2 3 4 5 6 7 https://www.drugs.com/international/normethandrone.html
- 1 2 3 4 5 Friedl KE (1990). "Reappraisal of the health risks associated with the use of high doses of oral and injectable androgenic steroids". NIDA Res. Monogr. 102: 142–77. PMID 1964199.
- 1 2 3 4 5 H.-L. Krüskemper (22 October 2013). Anabolic Steroids. Elsevier. pp. 10–. ISBN 978-1-4832-6504-9.
- 1 2 3 4 5 Ferin J (1956). "A new substance with progestational activity; comparative assays in ovariectomized women; clinical results". Acta Endocrinol. 22 (4): 303–17. doi:10.1530/acta.0.0220303. PMID 13354223.
- 1 2 Lundberg, P O (1962). "Migraine Prophylaxis with Progestogens". European Journal of Endocrinology. 40 (4 Suppl): S5–S22. doi:10.1530/acta.0.040S0005. ISSN 0804-4643.
- 1 2 Delorimier AA, Gordan GS, Lowe RC, Carbone JV (1965). "Methyltestosterone, Related Steroids, and Liver Function". Arch. Intern. Med. 116: 289–94. doi:10.1001/archinte.1965.03870020129023. PMID 14315662.
- 1 2 3 Brueggemeier, Robert W. (2006). "Sex Hormones (Male): Analogs and Antagonists": 42. doi:10.1002/3527600906.mcb.200500066.
- ↑ Erich Heftmann (1970). Steroid Biochemistry. Academic Press. p. 72.
Normethandrone (Fig. 49) is a 19-nortestosterone derivative having progestational as well as androgenic and anabolic activity.
- 1 2 United States. Patent Office (1957). Official Gazette of the United States Patent Office. U.S. Patent Office.
- 1 2 3 Unlisted Drugs. Pharmaceutical Section, Special Libraries Association. 1982.
Batynid. C. Each dragee contains: normethandrone, 5 mg.; and methylestradiol, 0.3 mg. E. (Formerly) Gynaekosid. M. Boehringer Biochemia, Florence. A. Estrogenic; Rx of secondary amenorrhea. R. Notiz Med Farm 32;295, Nov-Dec 81.
- ↑ Begni-Calvet, D. (1959). "Two properties of methylestrenolone (17-alpha-methyl-19-nortestosterone): Its effectiveness in the treatment of dysmenorrhea, its anabolic action". Gynecologie pratique. 10: 261–272. PMID 13798272.
- ↑ Servais, J. (1973). "A clinical study of cases of psychosexual disturbances in men treated by a libido inhibitor: Methylestrenolone". Archives of Sexual Behavior. 2 (4): 387–390. doi:10.1007/BF01541012. ISSN 0004-0002.
- 1 2 Akingba JB, Ayodeji EA (February 1966). "Amenorrhea as a leading symptom of choriocarcinoma". J Obstet Gynaecol Br Commonw. 73 (1): 153–5. doi:10.1111/j.1471-0528.1966.tb05137.x. PMID 5948541.
- ↑ Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 379–. ISBN 978-3-642-66353-6.
- 1 2 3 Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- ↑ Behre HM, Kliesch S, Lemcke B, von Eckardstein S, Nieschlag E (December 2001). "Suppression of spermatogenesis to azoospermia by combined administration of GnRH antagonist and 19-nortestosterone cannot be maintained by this non-aromatizable androgen alone". Hum. Reprod. 16 (12): 2570–7. PMID 11726576.
- ↑ Delettré J, Mornon JP, Lepicard G, Ojasoo T, Raynaud JP (January 1980). "Steroid flexibility and receptor specificity". J. Steroid Biochem. 13 (1): 45–59. doi:10.1016/0022-4731(80)90112-0. PMID 7382482.
- ↑ Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Res. 38 (11 Pt 2): 4186–98. PMID 359134.
- ↑ Detlef Thieme; Peter Hemmersbach (18 December 2009). Doping in Sports. Springer Science & Business Media. pp. 470–. ISBN 978-3-540-79088-4.
- ↑ William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 444–454, 533. ISBN 978-0-9828280-1-4.
- ↑ Fragkaki AG, Angelis YS, Tsantili-Kakoulidou A, Koupparis M, Georgakopoulos C (May 2009). "Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine". J. Steroid Biochem. Mol. Biol. 115 (1–2): 44–61. doi:10.1016/j.jsbmb.2009.02.016. PMID 19429460.
- ↑ Schjølberg, T. H. (2013). In Vitro Synthesis of Metabolites of three Anabolic Androgenic Steroids, by Human Liver Microsomes (Master's thesis, Institutt for bioteknologi). https://brage.bibsys.no/xmlui/handle/11250/246018
- 1 2 Die Gestagene. Springer-Verlag. 27 November 2013. pp. 12–13, 282. ISBN 978-3-642-99941-3.
- 1 2 3 4 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 888–. ISBN 978-1-4757-2085-3.
- 1 2 3 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 202–. ISBN 978-94-011-4439-1.
- ↑ Martin Negwer; Hans-Georg Scharnow (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 1831. ISBN 978-3-527-30247-5.