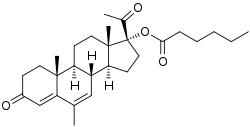
Megestrol caproate
 | |
| Clinical data | |
|---|---|
| Synonyms | MGC; Megestrol hexanoate; Megestrol 17α-caproate; 17α-Hydroxy-6-dehydro-6-methylprogesterone 17α-caproate; 17α-Hydroxy-6-methylpregna-4,6-diene-3,20-dione 17α-caproate |
| Drug class | Progestin; Progestogen; Progestogen ester |
| Identifiers | |
| |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C28H40O4 |
| Molar mass | 440.624 g/mol |
| 3D model (JSmol) | |
| |
| |
Megestrol caproate, abbreviated as MGC, is a progestin medication which was never marketed.[1][2] It was developed in Russia in 2002.[1] In animals, MGC shows 10-fold higher progestogenic activity compared to progesterone when both are administered via subcutaneous injection.[1] In addition, MGC has no androgenic, anabolic, or estrogenic activity.[1] The medication was suggested as a potential contraceptive and therapeutic agent.[1]
Chemistry
Megestrol caproate, also known as megestrol 17α-caproate, as well as 17α-hydroxy-6-dehydro-6-methylprogesterone 17α-caproate or as 17α-hydroxy-6-methylpregna-4,6-diene-3,20-dione 17α-caproate, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[1][2] It is the C17α caproate (hexanoate) ester of megestrol.[1][2] Closely related medications include megestrol acetate (MGA; megestrol 17α-acetate), acetomepregenol (megestrol 3β,17α-diacetate), and cymegesolate (megestrol 17α-acetate 3β-cypionate).[3][4][5][6] In addition to MGA, analogues of MGC include chlormadinone caproate, gestonorone caproate, hydroxyprogesterone caproate, medroxyprogesterone caproate, and methenmadinone caproate.
References
- 1 2 3 4 5 6 7 Pirzada OL (June 2002). "Effect of megestrol caproate on the reproductive function of laboratory animals" (PDF). Bull. Exp. Biol. Med. 133 (6): 574–6. doi:10.1023/A:1020233925626. PMID 12447469.
- 1 2 3 Grinenko, G. S.; Kadatskii, G. M.; Korkhov, V. V.; Boikova, V. V. (1981). "Preparation of 6-methylpregna-4,6-diene-3?,17?-diol-20-one 17-caproate and its influence on the reproductive function in rats" (PDF). Pharmaceutical Chemistry Journal. 15 (10): 718–720. doi:10.1007/BF00765383. ISSN 0091-150X.
- ↑ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 595, 664, 657. ISBN 978-1-4757-2085-3.
- ↑ Grinenko, G. S.; Popova, E. V.; Korkhov, V. V.; Lesik, E. A.; Petrosyan, M. A.; Topil'skaya, N. I. (March 2000). "Synthesis and biological activity of 17α-acetoxy-3β-phenylpropionyloxy-6-methylpregna-4,6-dien-20-one". Pharmaceutical Chemistry Journal. 34 (3): 113–114. doi:10.1007/BF02524577. ISSN 1573-9031.
Note that 3,17-diacetoxy-6-methylpregna-4,6-dien-20-one (1b), a structural analog of compound 1a, is certified in Russia under the trade name acetomepregnol and recommended for therapeutic purposes in gynecological practice and as a contraceptive preparation in combination with estrogens [4].
- ↑ De-Wei Z (1982). "Research activities in the field of oral contraceptives in the People's Republic of China". Acta Obstet Gynecol Scand Suppl. 105: 51–60. PMID 6952745.
- ↑ Yang, Yi-chien; Gu, Xi-gen; Li, Shu-xiang (1982). "Antifertility Effect of a Long-Acting Progestin (3-Cyclopentyl Propionate of Megestrol Acetate): Prematurity of the Endometrium and Accompanying Changes of Uteroglobin and Progesterone in Uterine Fluid": 335–342. doi:10.1007/978-3-642-67890-5_22.