Testolactone
 | |
| Clinical data | |
|---|---|
| Trade names | Teslac |
| Synonyms | 13-Hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid δ-lactone; SQ-9538; Fludestrin; NSC-12173; NSC-23759 |
| AHFS/Drugs.com | Consumer Drug Information |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Aromatase inhibitor; Antiestrogen |
| ATC code |
|
| Pharmacokinetic data | |
| Protein binding | ~85% |
| Metabolism | Liver |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.012.304 |
| Chemical and physical data | |
| Formula | C19H24O3 |
| Molar mass | 300.39 g/mol |
| 3D model (JSmol) | |
| |
| | |
Testolactone (INN, USAN) (brand name Teslac) is a non-selective, irreversible, steroidal aromatase inhibitor which is used as an antineoplastic drug to treat advanced-stage breast cancer.[1][2][3][4] The drug was discontinued in 2008 and is no longer available for medical use.[4] However, it has been reported to still be marketed in the United States by Bristol-Myers Squibb under the brand name Teslac.[5]
Medical uses
This drug is mainly used for treating various types of breast cancer in women who have been through menopause or whose ovaries no longer function.[6] It works by blocking the production of estrogen, which helps prevent the growth of breast cancers that are activated by estrogen. It may also prevent tumor cells from being activated by other hormones.[6] It also has been used to postpone precocious puberty because of its ability to block estrogen production.[7] In addition, testolactone has been used in the treatment of gynecomastia.[8]
Side effects
The most common side effects include:
- Abnormal skin sensations
- Aches of the legs and arms
- General body discomfort
- Hair loss
- Loss of appetite
- Nausea
- Redness of the tongue
- Vomiting
Pharmacology
The principal action of testolactone is reported to be inhibition of aromatase activity and the reduction in estrogen synthesis that follows. Androstenedione, a 19-carbon steroid hormone produced in the adrenal glands and the gonads, is where estrone synthesis originates and is the source of estrogen in postmenopausal women. In vitro studies report that the aromatase inhibition may be noncompetitive and irreversible, and could possibly account for the persistence of this drug's effect on estrogen synthesis after drug withdrawal.[2]
In addition to its activity as an aromatase inhibitor, testolactone also reportedly possesses some anabolic activity and weak androgenic activity via binding to and activation of the androgen receptor (AR).[4] However, its affinity for the AR is very low; in one study, it showed 0.0029% of the affinity of the anabolic steroid metribolone (100%) for the human AR (Ki = 41 µM and 1.18 nM, respectively).[9] In accordance, androgenic side effects such as hirsutism, acne, and voice changes have been reported in no women in clinical trials with testolactone.[10]
Chemistry
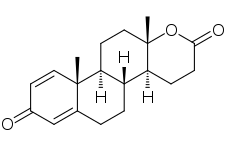
Testolactone, also known as 13-hydroxy-3-oxo-13,17-secoandrosta-1,4-dien-17-oic acid δ-lactone, is a synthetic 18-oxasteroid and a D-homo-18-oxo analogue of androstenedione (androst-4-en-3,17-dione), with a six-membered lactone ring in place of the five-membered carbocyclic D-ring.[4][1]
References
- 1 2 George W.A Milne (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 935–. ISBN 978-1-351-78989-9.
- 1 2 Testolactone at DrugBank.ca
- ↑ Dunkel L (July 2006). "Use of aromatase inhibitors to increase final height". Mol. Cell. Endocrinol. 254-255: 207–16. doi:10.1016/j.mce.2006.04.031. PMID 16766117.
- 1 2 3 4 Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1362–. ISBN 978-1-60913-345-0.
- ↑ https://www.drugs.com/international/testolactone.html
- 1 2 Testolactone facts and comparisons at Drugs.com
- ↑ Carel, J.-C.; Lahlou, N; Roger, M; Chaussain, JL (2004). "Precocious puberty and statural growth". Human Reproduction Update. 10 (2): 135–47. doi:10.1093/humupd/dmh012. PMID 15073143.
- ↑ Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1206–. ISBN 978-0-7817-1750-2.
- ↑ Eil C, Edelson SK (July 1984). "The use of human skin fibroblasts to obtain potency estimates of drug binding to androgen receptors". J. Clin. Endocrinol. Metab. 59 (1): 51–5. doi:10.1210/jcem-59-1-51. PMID 6725525.
- ↑ Aurel Lupulescu (24 October 1990). Hormones and Vitamins in Cancer Treatment. CRC Press. pp. 64–. ISBN 978-0-8493-5973-6.