Estradiol benzoate
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation |
/ˌɛstrəˈdaɪoʊl ES-trə-DY-ohl BEN-zoh-ayt |
| Trade names | Agofollin, Benovocyclin, Benzofoline, Dimenformon, Progynon-B, many others |
| Synonyms | EB; E2B; Oestradiol benzoate; 17β-Estradiol-3-benzoate; NSC-9566; Benzhormovarine, Difollisterol, Follicormon, Follidimyl, Follidrinbensoat, Oestro-Vitis, Oestroform |
| Routes of administration | Intramuscular injection |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High |
| Metabolism | Cleavage via esterases in the liver, blood, and tissues[1][2] |
| Metabolites | Estradiol, benzoic acid, and metabolites of estradiol[1][2] |
| Duration of action |
IM (0.3–1.7 mg): 2–3 days[3][4] IM (5 mg): 4–5 days[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.000.040 |
| Chemical and physical data | |
| Formula | C25H28O3 |
| Molar mass | 376.488 g/mol |
| 3D model (JSmol) | |
| |
| |
Estradiol benzoate, sold under the brand name Progynon-B among others, is a medication which is used in hormone therapy for menopausal symptoms and low estrogen levels in women, in hormone therapy for transgender women, and in the treatment of gynecological disorders.[4][6][7] It is also used in the treatment of prostate cancer in men.[4] Estradiol benzoate is used in veterinary medicine as well.[8][9] When used clinically, the medication is given by injection into muscle usually two to three times per week.[4][6][10]
Side effects of estradiol benzoate include breast tenderness, breast enlargement, nausea, headache, and fluid retention.[11] Estradiol benzoate is a synthetic estrogen and hence is an agonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[1][2] It is an estrogen ester and a prodrug of estradiol in the body.[1][2] Because of this, it is considered to be a natural and bioidentical form of estrogen.[1]
Estradiol benzoate was discovered in 1933 and was introduced for medical use in 1936.[1][12][13][14] It was the first estradiol ester to be discovered or marketed, and was one of the first estrogens to be introduced for medical use.[13][14] Along with estradiol dipropionate, it was among the most widely used esters of estradiol for many years following its introduction.[15] However, in the 1950s, longer-acting estradiol esters that necessitate less frequent injections, such as estradiol valerate and estradiol cypionate, were developed, and have since mostly superseded estradiol benzoate.[5] Nonetheless, estradiol benzoate remains widely available throughout the world.[9] It is no longer available for medical use in the United States.[16]
Medical uses
The medical uses of estradiol benzoate are the same as those of estradiol and other estrogens.[4][6] Estradiol benzoate is used in hormone therapy for the treatment of menopausal symptoms such as hot flashes and vaginal atrophy and in the treatment of hypoestrogenism and delayed puberty due to hypogonadism or other causes in women.[4][6] It is also used in hormone therapy for transgender women.[7] Aside from hormone therapy, estradiol benzoate is used in the treatment of gynecological disorders such as menstrual disorders, dysfunctional uterine bleeding, and breast engorgement.[4][6] In addition, it is used as a form of high-dose estrogen therapy in the palliative treatment of prostate cancer in men.[4]
Estradiol benzoate has a relatively short duration of action, and is administered by intramuscular injection usually two to three times per week.[4][6] It is used in the treatment of menopausal symptoms at a dosage of 1 to 1.66 mg initially and 0.33 to 1 mg for maintenance two times per week, and in the treatment of hypoestrogenism and delayed puberty at a dosage of 1.66 mg two to three times per week.[4] The dosage used in hormone therapy for transgender women is 0.5 to 1.5 mg two to three times per week.[7] In the treatment of prostate cancer, estradiol benzoate is used at a dosage of 1.66 mg three times per week (for a total of 5 mg per week).[4]
Available forms
Estradiol benzoate is and has been available as an oil solution for intramuscular injection provided as vials and ampoules at concentrations of 0.2, 1, 2, 5, and 10 mg/mL, 50 mg/2 mL, and 5 mg/100 mL.[17][18]
Side effects
The side effects of estradiol benzoate are the same as those of estradiol. Examples of such side effects include breast tenderness and enlargement, nausea, bloating, edema, headache, and melasma.[11]
Pharmacology
Pharmacodynamics
Estradiol benzoate is an estradiol ester, or a prodrug of estradiol.[1][2] As such, it is an estrogen, or an agonist of the estrogen receptors.[1][2] Estradiol benzoate has very low affinity for the ERs, on the order of 100-fold less than that of estradiol.[19] As such, estradiol benzoate is regarded as essentially inactive in terms of estrogenic effect itself, acting solely as a prodrug to estradiol.[2] Estradiol benzoate is of about 38% higher molecular weight than estradiol due to the presence of its C3 benzoate ester.[20][9] Because estradiol benzoate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[1]
| Estrogen | Type | EPD (14 days) | Duration | |
|---|---|---|---|---|
| Estradiol benzoate | Bioidentical | 25–30 mg | 5 mg ≈ 5 days | |
| Estradiol dipropionate | Bioidentical | 25–30 mg | 5 mg ≈ 5–8 days | |
| Estradiol valerate | Bioidentical | 20 mg | 10 mg ≈ 14 days | |
| Estradiol cypionate | Bioidentical | 25–30 mg | 5 mg ≈ 14 days | |
| Polyestradiol phosphate | Bioidentical | 40–60 mg | 40 mg ≈ 28 days | |
| Diethylstilbestrol | Synthetic | 20 mg | 3 mg ≈ 3 days | |
| Diethylstilbestrol dipropionate | Synthetic | 15 mg | 2.5 mg ≈ 5 days | |
| Addendum: An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month.[21] Notes: All of the estrogens are by intramuscular injection. Abbreviations: EPD = Endometrial proliferation dose. Miscellaneous: Direct link to table. Sources: [22][23] | ||||
Pharmacokinetics
Following administration, estradiol benzoate acts as a prodrug of estradiol via cleavage by esterases into estradiol and the natural fatty acid benzoic acid.[2] This cleavage occurs not only in the liver, but also in the blood and in tissues.[1][2] Esters of estradiol like estradiol benzoate are readily hydrolyzed to estradiol, but have an extended duration when administered in oil via intramuscular injection due to a depot effect afforded by their fatty acid ester moiety.[2] The duration of action of estradiol benzoate at typical clinical doses by intramuscular injection (e.g., 0.33–1.66 mg) is said to be 2 to 3 days.[3][4] In contrast to the intramuscular route, the duration of estradiol benzoate is not prolonged if it is administered directly into the circulation via intravenous injection.[25] Similarly to estradiol valerate and estradiol acetate, estradiol benzoate is also active with oral administration.[3] However, it is not marketed in any preparation for use by this route.[17]
A single dose of 2.5 mg estradiol benzoate by intramuscular injection was found to produce plasma estradiol levels of greater than 400 pg/mL, measured 24 hours post-injection, in a group of patients with minimal baseline levels of estradiol (due to GnRH analogue therapy with triptorelin).[26]
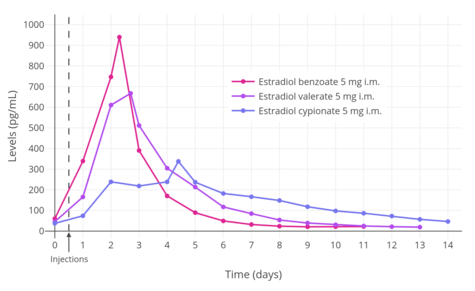
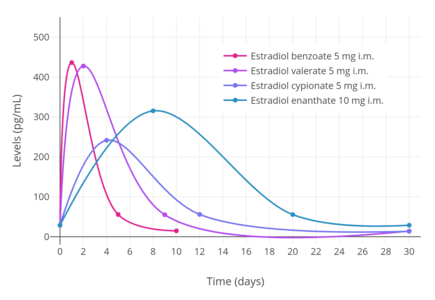
A single intramuscular injection of 5 mg estradiol benzoate has been found to result in peak circulating concentrations of 940 pg/mL estradiol and 343 pg/mL estrone, which occurred at about 2 days post-injection.[5] Compared to two other commonly used estradiol esters, estradiol benzoate had the shortest duration, at approximately 4 to 5 days, whereas estradiol valerate and estradiol cypionate were found to last for 7 to 8 days and 11 days, respectively.[5] This is because estradiol benzoate has a shorter and less bulky fatty acid chain, and in relation to this, is comparatively less lipophilic.[2] For a given estradiol ester, the shorter or less bulky the fatty acid chain is, the less lipophilic, shorter-lasting, and less uniform/plateau-like the resultant levels of estradiol are as well as the higher (and hence more spike-like) the peak/maximal levels are.[2]
| Estrogen | Peak levels | Time to peak | Duration |
|---|---|---|---|
| Estradiol benzoate | E2: 940 pg/mL E1: 343 pg/mL | E2: 1.8 days E1: 2.4 days | 4–5 days |
| Estradiol cypionate | E2: 338 pg/mL E1: 145 pg/mL | E2: 3.9 days E1: 5.1 days | 11 days |
| Estradiol valerate | E2: 667 pg/mL E1: 324 pg/mL | E2: 2.2 days E1: 2.7 days | 7–8 days |
Chemistry
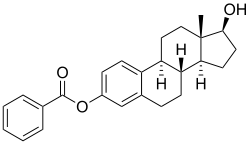
Estradiol benzoate is a synthetic estrane steroid and the C3 benzoate (phenylcarboxylate) ester of estradiol.[9][20][27] It is also known as estradiol 3-benzoate or as estra-1,3,5(10)-triene-3,17β-diol 3-benzoate.[9][20][27] Two estradiol esters that are related to estradiol benzoate are estradiol dipropionate, the C3,17β dipropionate ester of estradiol, and estradiol acetate, the C3 acetate ester of estradiol.
History
Estradiol benzoate was one of the first estrogens to be developed and marketed.[13][14] In 1931, Adolf Butenandt found that the benzoic acid ester of estrone had a prolonged duration of action.[28][29] Schwenk and Hildebrant discovered estradiol via reduction of estrone in 1933, and they proceeded to synthesize estradiol benzoate from estradiol the same year.[1][12] Estradiol benzoate was patented by Schering in 1933, and then by Schering-Kahlbaum in 1936 in an oil preparation for injectable use, and was introduced later that year in 1936 under the brand name Progynon-B.[13][14][17] Following its introduction, estradiol benzoate, along with estradiol dipropionate, were the most widely used esters of estradiol for many years.[15] However, estradiol valerate and estradiol cypionate, which are longer-acting esters that require less frequent administration, were developed and introduced in the 1950s, and have since largely superseded estradiol benzoate and estradiol dipropionate.[5]
Society and culture
Generic names
Estradiol benzoate is the generic name of the drug and its INN, BANM, and JAN, while oestradiol benzoate was formerly its BANM.[8][9][20][27]
Brand names
The major brand name of estradiol benzoate is Progynon-B.[9][20][27] It has also been sold under a variety of other brand names including Agofollin, Benovocyclin, Benzofoline, Dimenformon, and Ovocylin Benzoate, among many others.[9][20][27]
Availability
Estradiol benzoate is available in Europe and in other parts of the world.[9][17] It was previously available for medical use in the United States, but is no longer marketed in this country.[9][16][17][30] However, it is approved and marketed in the United States for veterinary use as a subdermal implant both alone and in combination with the androgen/anabolic steroid trenbolone acetate (brand names Celerin and Synovex, respectively).[16][31][32] Outside of the United States, estradiol benzoate is also marketed in combination with progesterone for use as an intramuscular injection.[8][33]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 8–. ISBN 978-3-642-58616-3.
- 1 2 3 4 5 6 7 8 9 10 11 12 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 3 H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 62–. ISBN 978-1-4612-5525-3.
- 1 2 3 4 5 6 7 8 9 10 11 12 "NNR: Products Recently Accepted by the A. M. A. Council on Pharmacy and Chemistry". Journal of the American Pharmaceutical Association (Practical Pharmacy ed.). 10 (11): 692–694. 1949. doi:10.1016/S0095-9561(16)31995-8. ISSN 0095-9561.
- 1 2 3 4 5 6 7 8 Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
- 1 2 3 4 5 6 Swyer GI (April 1959). "The oestrogens" (PDF). Br Med J. 1 (5128): 1029–31. doi:10.1136/bmj.1.5128.1029. PMC 1993181. PMID 13638626.
- 1 2 3 Gianna E. Israel (March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 64–. ISBN 978-1-56639-852-7.
- 1 2 3 https://www.drugs.com/international/estradiol.html
- 1 2 3 4 5 6 7 8 9 10 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 406. ISBN 978-3-88763-075-1.
- ↑ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 388–. ISBN 978-92-832-1291-1.
- 1 2 Amit K. Ghosh (23 September 2010). Mayo Clinic Internal Medicine Board Review. OUP USA. pp. 222–. ISBN 978-0-19-975569-1.
- 1 2 Miescher K, Scholz C, Tschopp E (1938). "The activation of female sex hormones: Mono-esters of alpha-oestradiol". Biochem. J. 32 (8): 1273–80. PMC 1264184. PMID 16746750.
- 1 2 3 4 Enrique Raviña; Hugo Kubinyi (16 May 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 175. ISBN 978-3-527-32669-3. Retrieved 20 May 2012.
- 1 2 3 4 Folley SJ (December 1936). "The effect of oestrogenic hormones on lactation and on the phosphatase of the blood and milk of the lactating cow" (PDF). The Biochemical Journal. 30 (12): 2262–72. PMC 1263335. PMID 16746289.
- 1 2 Schwartz MM, Soule SD (1955). "Estradiol 17-beta-cyclopentylpropionate, a longacting estrogen". Am. J. Obstet. Gynecol. 70 (1): 44–50. doi:10.1016/0002-9378(55)90286-6. PMID 14388061.
- 1 2 3 Richard Witherspoon (1 June 1994). Presidents List of Articles Which May Be Designated Or Modified As Eligible Articles for Purposes of the U.S. Generalized System of Preferences. DIANE Publishing. pp. 64–. ISBN 978-0-7881-1433-5.
- 1 2 3 4 5 A. Kleemann; J. Engel; B. Kutscher; D. Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1167–1174. ISBN 978-3-13-179525-0.
- ↑ Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 150, 349, 370. ISBN 978-3-7692-2114-5.
- ↑ Bovee TF, Helsdingen RJ, Rietjens IM, Keijer J, Hoogenboom RL (2004). "Rapid yeast estrogen bioassays stably expressing human estrogen receptors alpha and beta, and green fluorescent protein: a comparison of different compounds with both receptor types". J. Steroid Biochem. Mol. Biol. 91 (3): 99–109. doi:10.1016/j.jsbmb.2004.03.118. PMID 15276617.
- 1 2 3 4 5 6 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 897–. ISBN 978-1-4757-2085-3.
- ↑ Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ↑ Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Lehrbuch der Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 508–. ISBN 978-3-662-00526-2.
- ↑ Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 212–213. ISBN 978-3-662-00942-0.
- 1 2 3 4 Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ↑ Parkes AS (February 1938). "Effective Absorption of Hormones" (PDF). Br Med J. 1 (4024): 371–3. PMC 2085798. PMID 20781252.
- ↑ Vizziello G, D'Amato G, Trentadue R, Fanizza G (1993). "[Estradiol benzoate test in the study of pituitary block induced by triptorelin]". Minerva Ginecol (in Italian). 45 (4): 185–9. PMID 8506068.
- 1 2 3 4 5 A. D. Roberts (1991). Dictionary of Steroids: Chemical Data, Structures, and Bibliographies. CRC Press. p. 415. ISBN 978-0-412-27060-4. Retrieved 20 May 2012.
- ↑ A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 512–. ISBN 978-3-642-96158-8.
- ↑ Butenandt, A.; Hildebrandt, F. (1931). "Über ein zweites Hormonkrystallisat aus Schwangerenharn und seine physiologischen und chemischen Beziehungen zum krystallisierten Follikelhormon. [Untersuchungen über das weibliche Sexualhormon, 6. Mitteilung.]". Hoppe-Seyler's Zeitschrift für physiologische Chemie. 199 (4–6): 243–265. doi:10.1515/bchm2.1931.199.4-6.243. ISSN 0018-4888.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 16 November 2016.
- ↑ http://www.fda.gov/downloads/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/FOIADrugSummaries/ucm116143.pdf
- ↑ http://www.fda.gov/downloads/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/FOIADrugSummaries/UCM338208.pdf
- ↑ "Progesterone". Drugs.com. Retrieved 2018-07-31.