Estradiol undecylate
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation |
/ˌɛstrəˈdaɪɒl ES-trə-DY-ol un-DESS-il-ayt |
| Trade names | Progynon Depot 100, others |
| Synonyms | EU; E2U; Estradiol undecanoate; Estradiol unducelate; RS-1047; SQ-9993 |
| Routes of administration | Intramuscular injection[1] |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High |
| Metabolism | Cleavage via esterases in the liver, blood, and tissues[2][3] |
| Metabolites | Estradiol, undecanoic acid, and metabolites of estradiol[2][3] |
| Duration of action |
IM (20–30 mg): ≥1.7 months[4] IM (100 mg): >4 weeks[5][6][7] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.020.616 |
| Chemical and physical data | |
| Formula | C29H44O3 |
| Molar mass | 440.658 g/mol |
| 3D model (JSmol) | |
| |
| |
Estradiol undecylate, also known as estradiol undecanoate and sold under the brand name Progynon Depot 100 among others, is a medication which has been used in the treatment of prostate cancer in men.[8][9][10][5][11][1] It has also been used as a part of hormone therapy for transgender women.[12][13] Although estradiol undecylate has been used in the past, it has mostly or fully been discontinued and hence is now largely or entirely no longer available.[10][14] It is given by injection into muscle usually once a month.[1][5][11]
Side effects of estradiol undecylate in men include breast tenderness, breast development, feminization, sexual dysfunction, infertility, fluid retention, and cardiovascular issues.[5] Estradiol undecylate is a synthetic estrogen and hence is an agonist of the estrogen receptor (ER), the biological target of estrogens like estradiol.[3][2] It is an estrogen ester and a very long-lasting prodrug of estradiol in the body.[2][3] Because of this, it is considered to be a natural and bioidentical form of estrogen.[2]
Estradiol undecylate was discovered in 1954 and was introduced for medical use by 1956.[15][16][17][18] It has been used in Europe as a parenteral form of estrogen to treat men with prostate cancer, although not as frequently as polyestradiol phosphate.[10][11] It does not appear to have ever been available in the United States.[10][19]
Medical uses
Estradiol undecylate has been used in high doses (100 mg intramuscular injection every 3 weeks or once per month)[5][6] as a form of high-dose estrogen therapy to treat prostate cancer, but has since largely been superseded for this indication by newer agents with fewer adverse effects (e.g., gynecomastia and cardiovascular complications) like GnRH analogues and nonsteroidal antiandrogens.[1][20] Estradiol undecylate has also been used to treat breast cancer in women.[21] Along with estradiol valerate, estradiol cypionate, and estradiol benzoate, estradiol undecylate has been used as an intramuscular estrogen in feminizing hormone therapy for transgender women.[12][13]
Available forms
Estradiol undecylate was available as an oil solution for intramuscular injection provided in ampoules at a concentration of 100 mg/mL.[22][23]
Side effects
Estradiol undecylate has been used at high doses in the treatment of prostate cancer.[5] At these high doses, it was found to been associated with a considerable incidence of cardiovascular complications in elderly men with prostate cancer in one clinical study.[11][5][24] In a 6-month study of 21 men age 51 to 84 years (mean 68 years) with a life expectancy of less than 6 months given 100 mg/month intramuscular estradiol undecylate to treat advanced prostate cancer, a 67% (14/21) incidence of cardiovascular morbidity and 9.5% (2/21) incidence of cardiovascular mortality occurred (76%; 16/21 incidence total).[11][5][24] The cardiovascular morbidity included edema and thrombophlebitis (38%; 8/21), coronary heart disease (24%; 5/21), and deep vein thrombosis (4.8%; 1/21), while the cardiovascular mortality included a myocardial infarction (4.8%; 1/21) and a pulmonary embolism (4.8%; 1/21).[5][24] No incidence of cardiovascular toxicity occurred in the 300 mg/week intramuscular cyproterone acetate comparison group (0%; 0/21).[11][5][24] Other side effects of estradiol undecylate included gynecomastia (100%; 21/21) and erectile dysfunction (90%; 19/21).[5] These findings are in contrast to clinical studies of high-dose polyestradiol phosphate and transdermal estradiol for prostate cancer, in which minimal or no cardiovascular toxicity has been observed.[25][26][27][28] These studies have had much larger sample sizes and have been of better quality than the estradiol undecylate study.[25][26][27][28]
Overdose
Estrogens are relatively safe in the event of acute overdose. Estradiol undecylate has been used clinically at extremely high doses of up to 800 mg per month by intramuscular injection, given in divided doses of 100 mg injections twice per week.[13][29][30] For purposes of comparison, 100 mg per month estradiol undecylate has been found to produce maximal estradiol levels of about 500 pg/mL.[7]
Pharmacology
Pharmacodynamics
Esters of estradiol like estradiol undecylate are readily hydrolyzed prodrugs of estradiol, but have an extended duration when administered in oil via intramuscular injection due to a depot effect afforded by their fatty acid ester moiety.[3] As prodrugs of estradiol, estradiol undecylate and other estradiol esters are estrogens.[2][3] Estradiol undecylate is of about 62% higher molecular weight than estradiol due to the presence of its C17β undecylate ester.[8][10] Because estradiol undecylate is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[2]
Antigonadotropic activity
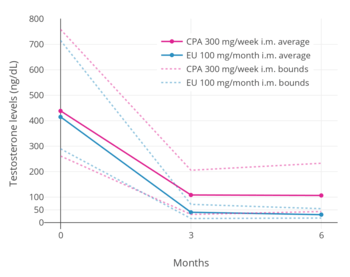
A phase III clinical trial comparing high-dose intramuscular cyproterone acetate (300 mg/week) and high-dose intramuscular estradiol undecylate (100 mg/month) in the treatment of prostate cancer found that estradiol undecylate suppressed testosterone levels into the castrate range (< 50 ng/dL)[31] within at least 3 months whereas testosterone levels with cyproterone acetate were significantly higher and above the castrate range even after 6 months of treatment.[5] With estradiol undecylate, testosterone levels fell from 416 ng/dL to 38 ng/mL (-91%) after 3 months and to 29.6 ng/dL (-93%) after 6 months, whereas with cyproterone acetate, testosterone levels fell from 434 ng/dL to 107 ng/mL (-75%) at 3 months and to 102 ng/mL (-76%) at 6 months.[5] In accordance, whereas estrogens are well-established as able to suppress testosterone levels into the castrate range at sufficiently high dosages,[32] progestogens like cyproterone acetate on their own are able to decrease testosterone levels only up to an apparent maximum of around 70 to 80%.[33][34]
Pharmacokinetics
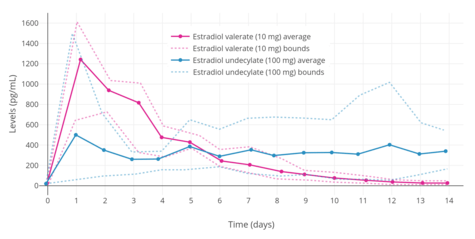
The pharmacokinetics of estradiol undecylate have been assessed in a few studies.[7][35][36] Following a single intramuscular injection of 100 mg estradiol undecylate in oil, mean levels of estradiol were about 500 pg/mL a day after injection and about 340 pg/mL 14 days after injection.[7] Levels of estradiol with intramuscular estradiol undecylate are very irregular and vary by as much as 10-fold between individuals.[7] However, the duration of estradiol undecylate is considerably prolonged relative to that of estradiol benzoate, estradiol valerate, and other estradiol esters.[7][37] A single injection of estradiol undecylate may remain effective for some months.[37]
Chemistry
Estradiol undecylate is a synthetic estrane steroid and the C17β undecylate (undecanoate) ester of estradiol.[8][9][10] It is also known as estradiol 17β-undecylate or as estra-1,3,5(10)-triene-3,17β-diol 17β-undecanoate.[9][10] A few related estradiol esters include estradiol decanoate, estradiol diundecylate, and estradiol diundecylenate.[8][9]
Estradiol undecylate is a relatively long-chain ester of estradiol. Its ester chain contains 11 carbon atoms. For comparison, the ester chains of estradiol acetate, estradiol valerate, and estradiol enantate have 2, 5, and 7 carbon atoms, respectively. As a result of its long ester chain, estradiol undecylate has by far the longest duration of all of these estradiol esters when administered via intramuscular injection.[7]
Estradiol undecylate shares the same undecylate ester as testosterone undecanoate, an androgen/anabolic steroid and very long-lasting testosterone ester.[8][9]
History
Estradiol undecylate was first described in the scientific literature, along with estradiol valerate, in 1954.[15] It was introduced for medical use by 1956.[16][17][18] Syntex applied for a patent for estradiol undecylate in 1958, which was granted in 1961 and was given a priority date of 1957.[22][38] Estradiol undecylate was introduced for medical use, but was subsequently discontinued.[10][39][23] It remained in use in France and Japan as late as 2009.[22][10]
Society and culture
Generic names
Estradiol undecylate is the generic name of the drug and its INN and USAN.[8][9][10][14] It is also spelled in some publications as estradiol unducelate and is also known as estradiol undecanoate.[7][8][9][10][14] In German, it is known under a variety of spellings including as estradiolundecylat, östradiolundecylat, östradiolundezylat, oestradiolundecylat, oestradiolundezylat, and others.[40] Estradiol undecylate is known by its former developmental code names RS-1047 and SQ-9993 as well.[8][9][10][14]
Brand names
The major brand name of estradiol undecylate is Progynon Depot 100.[8][9][10] It has also been marketed under a variety of other brand names including Delestrec, Depogin, Oestradiol-Retard Theramex, Primogyn Depot, and Progynon Depot, among others.[8][9][10][22]
Availability
Estradiol undecylate was available in the United States, Europe (including in France, Germany, Monaco, the Netherlands, Switzerland, and Monaco), and Japan.[10][22] However, is has been discontinued and is no longer available.[39][23]
Research
Estradiol undecylate was studied by Schering alone as an estrogen-only injectable contraceptive at a dose of 20 to 30 mg once a month.[4][41][42] It was effective, lacked breast and thromboembolic complications, lacked other side effects besides amenorrhea, and prevented ovulation for 1 to 3 months (mean 1.7 months) following a single dose.[4] However, endometrial hyperplasia was occasionally encountered, and the preparation was not further developed for this purpose due to the risks of endometrial hyperplasia and cancer associated with long-term unopposed estrogen therapy.[4]
Estradiol undecylate, in combination with norethisterone enantate (at doses of 5 to 10 mg and 50 to 70 mg, respectively), was studied by Schering as a combined injectable contraceptive and was found to be effective and well-tolerated, but ultimately was not marketed for this use.[43][42][4][44]
References
- 1 2 3 4 Christoph Zink (1 January 1988). Dictionary of Obstetrics and Gynecology. Walter de Gruyter. p. 85. ISBN 978-3-11-085727-6.
- 1 2 3 4 5 6 7 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- 1 2 3 4 5 6 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 3 4 5 Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- 1 2 R. S. Satoskar; S. D. Bhandarkar &nirmala N. Rege (1973). Pharmacology and Pharmacotherapeutics. Popular Prakashan. pp. 934–. ISBN 978-81-7991-527-1.
- 1 2 3 4 5 6 7 8 9 10 11 Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- 1 2 3 4 5 6 7 8 9 10 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 898–. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 8 9 10 A. D. Roberts (1991). Dictionary of Steroids: Chemical Data, Structures, and Bibliographies. CRC Press. p. 415. ISBN 978-0-412-27060-4. Retrieved 20 May 2012.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 405. ISBN 978-3-88763-075-1. Retrieved 20 May 2012.
- 1 2 3 4 5 6 Norman G, Dean ME, Langley RE, Hodges ZC, Ritchie G, Parmar MK, Sydes MR, Abel P, Eastwood AJ (2008). "Parenteral oestrogen in the treatment of prostate cancer: a systematic review". Br. J. Cancer. 98 (4): 697–707. doi:10.1038/sj.bjc.6604230. PMC 2259178. PMID 18268497.
- 1 2 Harry Benjamin (1966). The Transsexual Phenomenon. Julian Press. ISBN 978-0-446-82426-2.
- 1 2 3 Gianna E. Israel (March 2001). Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. pp. 64–. ISBN 978-1-56639-852-7.
- 1 2 3 4 https://www.drugs.com/international/estradiol.html
- 1 2 Wied GL (January 1954). "[Estradiol valerate and estradiol undecylate, two new estrogens with prolonged action; comparison with estradiol benzoate]". Geburtshilfe Und Frauenheilkunde. 14 (1): 45–52. PMID 13142295.
- 1 2 Halkerston ID, Hillman J, Palmer D, Rundle A (1956). "Changes in the excretion pattern of neutral 17-ketosteroids during oestrogen administration to male subjects". J. Endocrinol. 13 (4): 433–8. doi:10.1677/joe.0.0130433. PMID 13345960.
- 1 2 Gouygou C, Gueritee N, Pye A (1956). "[A fat-soluble, delayed estrogen : the estradiol undecylate]". Thérapie (in French). 11 (5): 909–17. PMID 13391788.
- 1 2 Bishop, P. M. F. (1958). Endocrine Treatment of Gynaecological Disorders. Modern Trends in Endocrinology, 231.
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 2 January 2018.
- ↑ Ernst Mutschler; Hartmut Derendorf (1995). Drug Actions: Basic Principles and Therapeutic Aspects. CRC Press. p. 609. ISBN 978-0-8493-7774-7. Retrieved 30 January 2013.
- ↑ Kennedy, B. J. (1967). Effect of massive doses of estradiol undecylate in advanced breast cancer. Cancer Chemother. Rep, 51, 491-495.
- 1 2 3 4 5 A. Kleemann; J. Engel; B. Kutscher; D. Reichert (14 May 2014). Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. pp. 1167–1174. ISBN 978-3-13-179525-0.
- 1 2 3 Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2098. ISBN 978-0-85369-840-1.
- 1 2 3 4 Wenderoth, U. K.; Jacobi, G. H. (1983). "Gonadotropin-releasing hormone analogues for palliation of carcinoma of the prostate". World Journal of Urology. 1 (1): 40–48. doi:10.1007/BF00326861. ISSN 0724-4983.
- 1 2 Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer—a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- 1 2 Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- 1 2 Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- 1 2 Langley RE, Cafferty FH, Alhasso AA, Rosen SD, Sundaram SK, Freeman SC, Pollock P, Jinks RC, Godsland IF, Kockelbergh R, Clarke NW, Kynaston HG, Parmar MK, Abel PD (April 2013). "Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09)". Lancet Oncol. 14 (4): 306–16. doi:10.1016/S1470-2045(13)70025-1. PMC 3620898. PMID 23465742.
- ↑ Asscheman H, Gooren LJ, Assies J, Smits JP, de Slegte R (June 1988). "Prolactin levels and pituitary enlargement in hormone-treated male-to-female transsexuals". Clin. Endocrinol. (Oxf). 28 (6): 583–8. doi:10.1111/j.1365-2265.1988.tb03849.x. PMID 2978262.
- ↑ Gazzeri R, Galarza M, Gazzeri G (December 2007). "Growth of a meningioma in a transsexual patient after estrogen-progestin therapy". N. Engl. J. Med. 357 (23): 2411–2. doi:10.1056/NEJMc071938. PMID 18057351.
- ↑ Fabian M. Saleh (11 February 2009). Sex Offenders: Identification, Risk Assessment, Treatment, and Legal Issues. Oxford University Press, USA. pp. 176–. ISBN 978-0-19-517704-6.
- ↑ Muhammad A. Salam (2003). Principles & Practice of Urology: A Comprehensive Text. Universal-Publishers. pp. 684–. ISBN 978-1-58112-412-5.
- ↑ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- ↑ Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clinical Endocrinology. 11 (5): 497–504. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881.
- ↑ Leyendecker G, Geppert G, Nocke W, Ufer J (May 1975). "Untersuchungen zur Pharmakokinetik von Östradiol-17β, Östradiol-benzoat, Östradiol-Valerianat un Östradiol-Undezylat bei der Frau: Der Verlauf der Konzentration von Östradiol-17β, Östron, LH und FSH im Serum" [Estradiol 17β, estrone, LH and FSH in serum after administration of estradiol 17β, estradiol benzoate, estradiol valeriate and estradiol undecylate in the female]. Geburtshilfe Frauenheilkd (in German). 35 (5): 370–4. PMID 1150068.
Estradiol 17β, estradiol benzoate, estradiol valerianate, and estradiol undecylate were injected intravenously and intramuscularly to postmenopausal woman and to female castrates. Equal doses were used corresponding to 20 mg of free estradiol 17β. Estradiol 17β, estrone, FSH and LH were measured in serum by radioimmunoassay before and after application of the hormone and the estradiol esters. Thus the depot effect of the different esters could be compared.
- ↑ Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
Clinical pharmacokinetic information (mainly in terms of plasma levels of estradiol) is available on the behaviour of a variety of intramuscularly administered estradiol esters such as the benzoate (3-6). valerate (6), unducelate (6) and enanthate (7). Only a few studies attempted to compare the pharmacokinetic profile of various esters; one investigation compared the profiles of estradiol benzoate, valerate and unducelate although at relatively high doses corresponding to the equivalent of 20 mg of estradiol (6). [...] The present data also indicate that the three estradiol esters never yielded elevated estradiol levels beyond 15 days following the administration of doses used therapeutically. These findings are in contrast with those reported by others (6) following the injection of large quantities (exceeding 20 mg) of estradiol valerate and benzoate. This seeming discrepancy underlines the importance of the dosage to be selected for pharmacokinetic investigations, which must be close to that intended for clinical (contraceptive) use.
- 1 2 Percy Roberts Wilde; Carey Franklin Coombs; Arthur J. Rendle Short (1959). The Medical Annual: A Year Book of Treatment and Practitioner's Index ... Publishing Science Group.
As in the case of progestogens the esters of oestradiol vary in the duration of their effect. Oestradiol benzoate is short-acting (three days to a week). Oestradiol valerianate is somewhat longer-acting, and oestradiol enanthate and undecylate have considerably more prolonged duration of effectiveness. The undecylate may remain effective for some months, and should not be employed, [...]
- ↑ https://patents.google.com/patent/US2990414A/en
- 1 2 http://www.micromedexsolutions.com/micromedex2/librarian/
- ↑ Franz v. Bruchhausen; Gerd Dannhardt; Siegfried Ebel; August W. Frahm, Eberhard Hackenthal, Ulrike Holzgrabe (2 July 2013). Hagers Handbuch der Pharmazeutischen Praxis: Band 8: Stoffe E-O. Springer-Verlag. pp. 84–. ISBN 978-3-642-57994-3.
- ↑ el-Mahgoub S, Karim M (February 1972). "Depot estrogen as a monthly contraceptive in nulliparous women with mild uterine hypoplasia". Am. J. Obstet. Gynecol. 112 (4): 575–6. doi:10.1016/0002-9378(72)90319-5. PMID 5008627.
- 1 2 Goldsmith, A., & Toppozada, M. (1983). Long-acting contraception. pp. 94-95 https://www.popline.org/node/423289
- ↑ Toppozada MK (1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ↑ Dr. S. S. Kadam (July 2007). Principles of Medicinal Chemistry Volume 2. Pragati Books Pvt. Ltd. pp. 381–. ISBN 978-81-85790-03-9.