Omacetaxine mepesuccinate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Synribo |
| AHFS/Drugs.com | Monograph |
| License data | |
| Pregnancy category |
|
| Routes of administration | Subcutaneous, intravenous infusion |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 50% |
| Metabolism | Mostly via plasma esterases |
| Elimination half-life | 6 hours |
| Excretion | Urine (≤15% unchanged) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ECHA InfoCard |
100.164.439 |
| Chemical and physical data | |
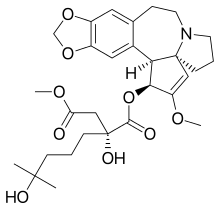
| Formula | C29H39NO9 |
| Molar mass | 545.62 g/mol |
| 3D model (JSmol) | |
| |
| |
Omacetaxine mepesuccinate (INN, trade names Synribo or Myelostat ), formerly named as homoharringtonine or HHT, is a pharmaceutical drug substance that is indicated for treatment of chronic myeloid leukemia (CML). It is a natural product first discovered in Cephalotaxus harringtonii, now manufactured by hemi-synthesis. It was approved by the US FDA in October 2012 for the treatment of adult patients with CML with resistance and/or intolerance to two or more tyrosine kinase inhibitors (TKIs).[1]
Medical uses
Omacetaxine/homoharringtonine is indicated for use as a treatment for patients with chronic myeloid leukaemia who are resistant or intolerant of tyrosine kinase inhibitors.[2][3][4]
In June 2009, results of a long-term open label Phase II study were published, which investigated the use of omacetaxine infusions in CML patients. After twelve months of treatment, about one third of patients showed a cytogenetic response.[5] A study in patients who had failed imatinib and who had the drug resistant T315I mutation achieved cytogenetic response in 28% of patients and hematologic response in 80% of patients, according to preliminary data.[6]
Phase I studies including a small number of patients have shown benefit in treating myelodysplastic syndrome (MDS, 25 patients)[7] and acute myelogenous leukaemia (AML, 76 patients).[8] Patients with solid tumors did not benefit from omacetaxine.[9]
Adverse effects
By frequency:[1][2]
Very common (>10% frequency):
- Diarrhoea
- Myelosuppression†
- Injection site reactions
- Nausea
- Fatigue
- Fever
- Muscle weakness
- Joint pain
- Headache
- Cough
- Hair loss
- Constipation
- Nosebleeds
- Upper abdominal pain
- Pain in the extremities
- Oedema
- Vomiting
- Back pain
- Hyperglycemia, sometimes extreme
- Gout
- Rash
- Insomnia
Common (1–10% frequency):
- Seizures
- Haemorrhage
† Myelosuppression, including: thrombocytopenia, anaemia, neutropenia and lymphopenia, in descending order of frequency.
Mechanism of action
Omacetaxine is a protein translation inhibitor. It inhibits protein translation by preventing the initial elongation step of protein synthesis. It interacts with the ribosomal A-site and prevents the correct positioning of amino acid side chains of incoming aminoacyl-tRNAs. Omacetaxine acts only on the initial step of protein translation and does not inhibit protein synthesis from mRNAs that have already commenced translation.[10]
References
- 1 2 "Synribo (omacetaxine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 18 February 2014.
- 1 2 "SYNRIBO (omacetaxine mepesuccinate) injection, powder, lyophilized, for solution [Cephalon, Inc.]". DailyMed. Cephalon, Inc. October 2012. Retrieved 18 February 2014.
- ↑ Sweetman, S, ed. (14 November 2012). "Omacetaxine Mepesuccinate". Martindale: The Complete Drug Reference. Medicines Complete. Pharmaceutical Press.
|access-date=requires|url=(help) - ↑ Lacroix, Marc (2014). Targeted Therapies in Cancer. Hauppauge , NY: Nova Sciences Publishers. ISBN 978-1-63321-687-7.
- ↑ Li, Y. F.; Deng, Z. K.; Xuan, H. B.; Zhu, J. B.; Ding, B. H.; Liu, X. N.; Chen, B. A. (2009). "Prolonged chronic phase in chronic myelogenous leukemia after homoharringtonine therapy". Chinese medical journal. 122 (12): 1413–1417. PMID 19567163.
- ↑ Quintás-Cardama, A.; Kantarjian, H.; Cortes, J. (2009). "Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa 2009". Cancer. 115 (23): 5382–5393. doi:10.1002/cncr.24601. PMID 19739234.
- ↑ Wu, L.; Li, X.; Su, J.; Chang, C.; He, Q.; Zhang, X.; Xu, L.; Song, L.; Pu, Q. (2009). "Effect of low-dose cytarabine, homoharringtonine and granulocyte colony-stimulating factor priming regimen on patients with advanced myelodysplastic syndrome or acute myeloid leukemia transformed from myelodysplastic syndrome". Leukemia & Lymphoma. 50 (9): 1461–7. doi:10.1080/10428190903096719. PMID 19672772.
- ↑ Gu, L. F.; Zhang, W. G.; Wang, F. X.; Cao, X. M.; Chen, Y. X.; He, A. L.; Liu, J.; Ma, X. R. (2010). "Low dose of homoharringtonine and cytarabine combined with granulocyte colony-stimulating factor priming on the outcome of relapsed or refractory acute myeloid leukemia". Journal of Cancer Research and Clinical Oncology. 137 (6): 997–1003. doi:10.1007/s00432-010-0947-z. PMID 21152934.
- ↑ Kantarjian, H. M.; Talpaz, M.; Santini, V.; Murgo, A.; Cheson, B.; O'Brien, S. M. (2001). "Homoharringtonine". Cancer. 92 (6): 1591–1605. doi:10.1002/1097-0142(20010915)92:6<1591::AID-CNCR1485>3.0.CO;2-U. PMID 11745238.
- ↑ Wetzler M, Segal D. Omacetaxine as an Anticancer Therapeutic: What is Old is New Again. Current Pharmaceutical Design 2011;17:59–64