Idarubicin
 | |
 | |
| Clinical data | |
|---|---|
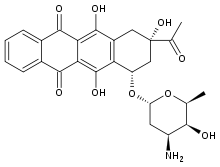
| Synonyms | 9-acetyl-7-(4-amino-5-hydroxy-6-methyl-tetrahydropyran-2-yl)oxy-6,9,11-trihydroxy-7,8,9,10-tetrahydrotetracene-5,12-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a691004 |
| Pregnancy category |
|
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 97% |
| Elimination half-life | 22 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H27NO9 |
| Molar mass | 497.494 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Idarubicin /ˌaɪdəˈruːbɪsɪn/ or 4-demethoxydaunorubicin is an anthracycline antileukemic drug. It inserts [1] itself into DNA and prevents DNA unwinding by interfering with the enzyme topoisomerase II. It is an analog of daunorubicin, but the absence of a methoxy group increases its fat solubility and cellular uptake.[2] Similar to other anthracyclines, it also induces histone eviction from chromatin.[3]
It belongs to the family of drugs called antitumor antibiotics.
It is currently combined with cytosine arabinoside as a first line treatment of acute myeloid leukemia.
It is distributed under the trade names Zavedos (UK) and Idamycin (USA).
References
- ↑ Miller JP, Stoodley RJ (2013). "Studies directed towards anthracyclinone syntheses: The use of d-glucose as a chiral auxiliary in asymmetric Diels–Alder reactions". J. Saudi Chem. Soc. 17: 29–42. doi:10.1016/j.jscs.2011.02.019.
- ↑ Package insert
- ↑ Pang B, Qiao X, Janssen L, Velds A, Groothuis T, Kerkhoven R, Nieuwland M, Ovaa H, Rottenberg S, van Tellingen O, Janssen J, Huijgens P, Zwart W, Neefjes J (2013). "Drug-induced histone eviction from open chromatin contributes to the chemotherapeutic effects of doxorubicin". Nature Communications. 4: 1908. doi:10.1038/ncomms2921. PMC 3674280. PMID 23715267.
External links
- Idarubicin bound to proteins in the PDB
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.