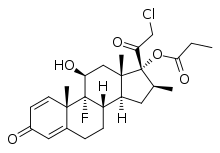
Clobetasol propionate
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Topical only |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.042.380 |
| Chemical and physical data | |
| Formula | C25H32ClFO5 |
| Molar mass | 466.97 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clobetasol propionate /kloʊˈbeɪtəsɒl/[1] is a corticosteroid of the glucocorticoid class used to treat various skin disorders including eczema and psoriasis. It is also highly effective for contact dermatitis caused by exposure to poison ivy/oak. Clobetasol belongs to US Class I (Europe: class IV) of the corticosteroids, making it one of the most potent available. It comes in shampoo, mousse, ointment and emollient cream presentations. It has very high potency and typically should not be used with occlusive dressings, or for extended continuous use (beyond two weeks). It is also used to treat several autoimmune diseases including alopecia areata, lichen sclerosus, and lichen planus.[2]
Uses
Clobetasol propionate is used for the treatment of various skin disorders including eczema, herpes labialis,[3] psoriasis, and lichen sclerosus. It is also used to treat several auto-immune diseases including alopecia areata, lichen planus (auto immune skin nodules), and mycosis fungoides (T-cell skin lymphoma). It is used as first-line treatment for both acute and chronic GVHD of the skin.
Clobetasol proprionate is used cosmetically by dark-skinned women for skin whitening, although this use is controversial. The U.S. Food and Drug Administration has not approved it for that purpose, and sales without a prescription are illegal in the U.S. Nonetheless, skin-whitening creams containing this ingredient can sometimes be found in ethnic beauty supply stores in New York City and on the internet. It is also sold internationally, and does not require a prescription in some countries. Whitening creams with clobetasol proprionate, such as Hyprogel, can make skin thin and easily bruised, with visible capillaries, and acne. It can also lead to hypertension, elevated blood sugar, suppression of the body’s natural steroids, and stretch marks, which may be permanent.[4]
Clobetasol propionate is, along with mercury and hydroquinone, "amongst the most toxic and most used agents in lightening products." Many products sold illegally have higher concentrations of clobetasol propionate than is permitted for prescription drugs.[5]
Contraindications
According to the California Environmental Protection Agency, clobetasol propionate should not be used by pregnant women, or women expecting to become pregnant soon, as studies with rats shows a risk of birth defects:[6]
"Studies in the rat following oral administration at dosage levels up to 50 mcg/kg per day revealed that the females exhibited an increase in the number of resorbed embryos and a decrease in the number of living fetuses at the highest dose. Pregnancy: Teratogenic Effects (i.e., possibility of causing abnormalities in fetuses): Pregnancy Category C: Clobetasol propionate has not been tested for teratogenicity when applied topically; however, it is absorbed percutaneously, and when administered subcutaneously it was a significant teratogen in both the rabbit and mouse. Clobetasol propionate has greater teratogenic potential than steroids that are less potent.There are no adequate and well-controlled studies of the teratogenic effects of clobetasol propionate in pregnant women. Temovate Cream and Ointment should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus."
Forms
Clobetasol propionate is marketed and sold worldwide under numerous names including Clobex, Clob-x (Colombia), Clovate, Clobet (Biolab Thailand) Clonovate (T.O. Chemicals, Thailand), Cormax (Watson, US), Haloderm (Switzerland, by ELKO Org), Pentasol (Colombia), Cosvate, Clop (Cadila Healthcare, India) , Propysalic (India), Temovate (US), Dermovate (GlaxoSmithKline, Canada, Pakistan, Portugal, Israel), Olux, ClobaDerm, Tenovate, Dermatovate, Butavate, Movate, Novate[7], Salac (Argentina), and Powercort.
See also
References
- ↑ "Clobetasol Propionate Topical Ointment 0.05% Information – Drug Encyclopedia". Kaiser Permanente.
- ↑ E. Fougera and Co. "CLOBETASOL PROPIONATE CREAM USP, 0.05% CLOBETASOL PROPIONATE OINTMENT USP, 0.05%<". NIH Daily Med.
- ↑ Hull, C; McKeough, M; Sebastian, K; Kriesel, J; Spruance, S (2009). "Valacyclovir and topical clobetasol gel for the episodic treatment of herpes labialis: A patient-initiated, double-blind, placebo-controlled pilot trial". Journal of the European Academy of Dermatology and Venereology. 23 (3): 263–267. doi:10.1111/j.1468-3083.2008.03047.x. PMID 19143902.
- ↑ Creams Offering Lighter Skin May Bring Risks By CATHERINE SAINT LOUIS, New York Times, JAN. 15, 2010
- ↑ Gbetoh MH, Amyot M. (1 July 2016). "Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada". Environ Res. 150: 403–10. doi:10.1016/j.envres.2016.06.030. PMID 27372064.
- ↑ Office of Environmental Health Hazard Assessment. "Chemicals Under Consideration For Possible Listing Via The "Formally Required To Be Labeled Or Identified" Mechanism". California Environmental Protection Agency.
- ↑ "Ulotka Novate / Clobetasoli propionas – leki i suplementy diety, dawkowanie, skład, zastosowanie, opis". DOZ.pl.