Methylene group
In organic chemistry, a methylene group is any part of a molecule that consists of two hydrogen atoms bound to a carbon atom, which is connected to the remainder of the molecule by a double bond. The group may be written =CH2, where the '=' denotes the double bond. This structural element is also called methylidene.
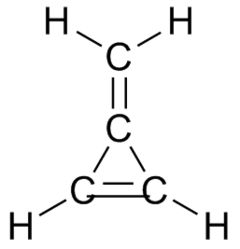
Many organic compounds are named and classified as if they were the result of substituting a methylene group for two adjacent hydrogen atoms of some parent molecule (even if they are not actually obtained that way). Thus, for example, methylenecyclopropene is named after cyclopropene.
The methylene group should be distinguished from the compound methylene, also called carbene, whose molecule is a methylene group all by itself. This compound is usually denoted CH
2. The singlet excited isomer of the methylene compound is systematically called methylidene.
The names "methylene group" or "bridging methylene" are also used for the substituent -CH
2-, that has the same composition as the methylene group but is connected by single bonds to two distinct atoms in the rest of the molecule. This unit may be better called "methylene bridge" or "methanediyl" to distinguish it from the doubly bonded methylene group. The distinction is often important, because the double bond is chemically different from two single bonds.
Activated methylene
The central carbon in 1,3-dicarbonyl compound is known as an activated methylene group. This is because, owing to the structure, the carbon is especially acidic and can easily be deprotonated to form a methylene group.[1]
See also
References