Thymus
| Thymus | |
|---|---|
 Thymus | |
| Details | |
| System | Immune system (Lymphatic system) |
| Lymph | tracheobronchial, parasternal |
| Identifiers | |
| Latin | Thymus |
| MeSH | D013950 |
| TA | A13.1.02.001 |
| FMA | 9609 |
| Anatomical terminology | |
The thymus is a specialized primary lymphoid organ of the immune system. Within the thymus, T cells mature. T cells are critical to the adaptive immune system, where the body adapts specifically to foreign invaders. The thymus is composed of two identical lobes and is located anatomically in the anterior superior mediastinum, in front of the heart and behind the sternum. Histologically, each lobe of the thymus can be divided into a central medulla and a peripheral cortex which is surrounded by an outer capsule. The cortex and medulla play different roles in the development of T cells. Cells in the thymus can be divided into thymic stromal cells and cells of hematopoietic origin (derived from bone marrow resident hematopoietic stem cells). Developing T cells are referred to as thymocytes and are of hematopoietic origin. Stromal cells include epithelial cells of the thymic cortex and medulla, and dendritic cells.
The thymus provides an inductive environment for development of T cells from hematopoietic progenitor cells. In addition, thymic stromal cells allow for the selection of a functional and self-tolerant T cell repertoire. Therefore, one of the most important roles of the thymus is the induction of central tolerance.
The thymus is largest and most active during the neonatal and pre-adolescent periods. By the early teens, the thymus begins to atrophy and thymic stroma is mostly replaced by adipose (fat) tissue. Nevertheless, residual T lymphopoiesis continues throughout adult life.
Structure
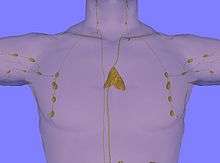
In children, the thymus is pinkish-gray, soft, and lobulated on its surfaces. At birth it is about 4–6 cm long, 2.5–5 cm wide, and about 1 cm thick.[1] It is made up of two lobes that meet in upper midline, that stretch from below the thyroid in the neck to as low as the cartilage of the fourth rib.[1] It lies beneath the sternum, rests on the pericardium, and is separated from the aortic arch and great vessels by a layer of fascia. The left brachiocephalic vein may even be embedded within the thymus.[1] In the neck, it lies on the front and sides of the trachea, behind the sternohyoidei and sternothyreoidei.[1]
The thymus enlarges during childhood, and atrophies at puberty. Unlike many other organs, the thymus is at its largest in childhood. The thymus reaches maximum weight (20 to 37 grams) by the time of puberty. The thymus of older people is scarcely distinguishable from surrounding fatty tissue. With aging the thymus slowly shrinks, eventually degenerating into tiny islands of fatty tissue. By the age of 75 years, the thymus weighs only 6 grams. In children the thymus is grayish-pink in colour and in adults it is yellow.
Microanatomy
The thymus consists of two lobes, merged in the middle, surrounded by a capsule that extends with blood vessels into the interior.[2] The lobes consist of a dense outer cortex and an inner less dense medulla.[2] The lobes are divided into smaller lobules 0.5-2mm diameter, between which extrude radiating insertions from the capsule along septa.[1]
The cortex is mainly made up of thymocytes, supported by a network of finely-branched epithelial reticular cells, which is continuous with a similar network in the medulla. This network forms an adventitia to the blood vessels, which enter the cortex via septa near the junction with the medulla.[1] The cortex is the location of the earliest events in thymocyte development, where T-cell receptor gene rearrangement and positive selection takes place.
In the medulla, the network of reticular cells is coarser than in the cortex, the lymphoid cells are relatively fewer in number, and there are concentric, nest-like bodies called Hassall's corpuscles. These are concentric, layered whorls of epithelial cells that increase in number throughout life.[1] They are the remains of the epithelial tubes, which grow out from the third pharyngeal pouches of the embryo to form the thymus.[3] In the center of the medullary portion there are very few vessels, and they are of minute size.
The medulla is the location of the latter events in thymocyte development. Thymocytes that reach the medulla have already successfully undergone T-cell receptor gene rearrangement and positive selection, and have been exposed to a limited degree of negative selection. The medulla is specialized to allow thymocytes to undergo additional rounds of negative selection to remove auto-reactive T cells from the mature repertoire. Transcriptional regulators AIRE and FEZ2 are expressed by the thymic medullary epithelium, and drives the transcription of organ-specific genes such as insulin to allow maturing thymocytes to be exposed to a more complex set of self-antigens than is present in the cortex.
- Micrograph of the thymus
 Structure of the thymus.
Structure of the thymus.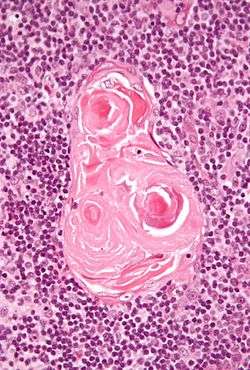 Micrograph showing a Hassall's corpuscle, found within the medulla of the thymus.
Micrograph showing a Hassall's corpuscle, found within the medulla of the thymus.
Blood and nerve supply
The arteries supplying the thymus are branches of the internal thoracic, and inferior thyroid arteries, with branches from the superior thyroid artery sometimes seen.[1] The branches reach the thymus and travel with the septa of the capsule into the area between the cortex and medulla, where they enter the thymus itself.[1]
The veins end in the left brachiocephalic vein, internal thoracic vein, and in the inferior thyroid veins.[1]
Lymphatic vessels travel only away from the thymus, accompanying the arteries and veins. These drain into the brachiocephalic, tracheobronchial and parasternal llymph nodes.[1]
The nerves supplying the thymus arise from the vagus nerve and the cervical sympathetic chain. Branches from the phrenic nerves reach the investing capsule, but do not enter into the thymus itself. Although present, the exact role of the nerve supply of the thymus is little understood.[1]
Variation
The two lobes differ slightly in size and may be united or separated.[4] Thymic tissue sometimes be found scattered on or around the gland.[1]
Development
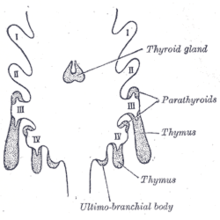
The thyomocytes and the epithelium of the thymus have different developmental origins. The epithelium of the thymus develops first, appearing as two flask-shape endodermal diverticula, which arise one on either side, from the third pharyngeal pouch, and extend outward and backward into the surrounding mesoderm and neural crest-derived mesenchyme in front of the ventral aorta. Here the thymocytes and epithelium meet and join with connective tissue. The pharyngeal opening of each diverticulum is soon obliterated, but the neck of the flask persists for some time as a cellular cord. By further proliferation of the cells lining the flask, buds of cells are formed, which become surrounded and isolated by the invading mesoderm. Additional portions of thymus tissue are sometimes developed from the fourth pharyngeal pouch.[5]
The epithelium forms fine lobules, and develops into a sponge-like structure. During this stage, hematopoietic bone-marrow precursors migrate into the thymus.[2] Normal development is dependent on the interaction between the epithelium and the hematopoietic thymocytes. Iodine is also necessary for thymus development and activity.[6]
Involution
The thymus continues to grow between birth and puberty and then begins to atrophy, called thymic involution.[2] The process accelerates after puberty, thymus decreases both in size and activity, and the tissue of the thymus is primarily replaced with fat (a phenomenon known as "organ involution").[2] The atrophy is due to the increased circulating level of sex hormones, and chemical or physical castration of an adult results in the thymus increasing in size and activity.[7] Fat cells are first visible in the walls between lobules, and then slowly spread throughout the cortex and then medulla.[2]
Function
In the two lobes, hematopoietic precursors from the bone-marrow, referred to as thymocytes, mature into T cells. Once mature, T cells emigrate from the thymus and make up the peripheral T cells responsible for directing many parts of the adaptive immune system. Loss of the thymus at an early age through genetic mutation (as in DiGeorge Syndrome[8]) results in severe immunodeficiency and subsequent high susceptibility to infection.[9]
Each T cell attacks a specific substance which it identifies with its receptor. T cells have receptors which are generated by randomly shuffling gene segments. Each T cell attacks a different antigen. T cells that attack the body's own proteins are eliminated in the thymus. Thymic epithelial cells express major proteins from elsewhere in the body. First, T cells undergo "Positive Selection", whereby the cell comes in contact with self-MHC, expressed by thymic epithelial cells; those with no interaction die by a lack of stimulatory signal. Second, the T cell undergoes "Negative Selection" by interacting with thymic dendritic cells, whereby T cells with a strong interaction with self-MHC and/or self-antigen die by induced apoptosis or are induced to become a regulatory T cell, to avoid autoimmunity. Those with intermediate affinity survive.
The stock of T-lymphocytes is built up in early life, so the function of the thymus is diminished in adults. It is largely degenerated in elderly adults and is barely identifiable, consisting mostly of fatty tissue. Involution of the thymus has been linked to loss of immune function in the elderly, susceptibility to infection and to cancer.
The ability of T cells to recognize foreign antigens is mediated by the T-cell receptor. The T-cell receptor undergoes genetic rearrangement during thymocyte maturation, resulting in each T cell bearing a unique T-cell receptor, specific to a limited set of peptide:MHC combinations. The random nature of the genetic rearrangement results in a requirement of central tolerance mechanisms to remove or inactivate those T cells which bear a T-cell receptor with the ability to recognise self-peptides.
- A rare population of hematopoietic progenitor cells enter the thymus from the blood, and expands by cell division to generate a large population of immature thymocytes.[10]
- Immature thymocytes each make distinct T-cell receptors by a process of gene rearrangement. This process is error-prone, and some thymocytes fail to make functional T-cell receptors, whereas other thymocytes make T-cell receptors that are autoreactive.[11]
- Immature thymocytes undergo a process of selection, based on the specificity of their T-cell receptors. This involves selection of T cells that are functional (positive selection), and elimination of T cells that are autoreactive (negative selection). The medulla of the thymus is the site of T Cell maturation.
| type: | functional (positive selection) | autoreactive (negative selection) |
| location: | cortex | medulla |
|
In order to be positively-selected, thymocytes will have to interact with several cell surface molecules, MHC/HLA, to ensure reactivity and specificity.[12] Positive selection eliminates (by apoptosis) weakly-binding cells and only takes strongly- or medium-binding cells. (Binding refers to the ability of the T-cell receptors to bind to either MHC class I/II or peptide molecules.) |
Negative selection is not 100% complete. Some autoreactive T cells escape thymic censorship, and are released into the circulation. Additional mechanisms of tolerance active in the periphery exist to silence these cells such as anergy, deletion, and regulatory T cells. If these peripheral tolerance mechanisms also fail, autoimmunity may arise. |
Cells that pass both levels of selection are released into the bloodstream to perform vital immune functions.
The thymus also secretes hormones and cytokines that regulate the maturation of T cells, including thymulin, thymopoietin, and thymosins.[2]
Clinical significance
The immune system is a multicomponent interactive system. It effectively protects the host from various infections. An improperly functioning immune system can cause discomfort, disease or even death. The type of malfunction falls into one or more of the following major groups: hypersensitivity or allergy, auto-immune disease, or immunodeficiency.
Hypersensitivity
Allergy results from an inappropriate and excessive immune response to common antigens. Substances that trigger an allergic response are called allergens. Allergies involve mainly IgE, antibodies, and histamine. Mast cells release the histamine. Sometimes an allergen may cause a sudden and severe, possibly fatal reaction in a sensitive individual; this is called anaphylaxis.
Immunodeficiency
As the thymus is the organ of T-cell development, any congenital defect in thymic genesis or a defect in thymocyte development can lead to a profound T cell deficiency in primary immunodeficiency disease. Defects that affect both the T cell and B cell lymphocyte lineages result in severe combined immunodeficiency (SCID). Acquired T cell deficiencies can also affect thymocyte development in the thymus.
DiGeorge syndrome
DiGeorge syndrome is a genetic disorder caused by the deletion of a small section of chromosome 22. This results in a midline congenital defect including thymic aplasia, or congenital deficiency of a thymus. Patients may present with a profound immunodeficiency disease, due to the lack of T cells. No other immune cell lineages are affected by the congenital absence of the thymus. DiGeorge syndrome is the most common congenital cause of thymic aplasia in humans. In mice, the nude mouse strain are congenitally thymic deficient. These mice are an important model of primary T cell deficiency.
SCID
Severe combined immunodeficiency syndromes (SCID) are group of rare congenital genetic diseases that result in combined T lymphocyte and B lymphocyte deficiencies. These syndromes are caused by defective hematopoietic progenitor cells which are the precursors of both B- and T cells. This results in a severe reduction in developing thymocytes in the thymus and consequently thymic atrophy. A number of genetic defects can cause SCID, including IL-7 receptor deficiency, common gamma chain deficiency, and recombination activating gene deficiency. The gene that codes for the enzyme called ADA (adenine deaminase), is located on chromosomes 20.
HIV/AIDS
The HIV virus causes an acquired T-cell immunodeficiency syndrome (AIDS) by specifically killing CD4+ T cells. Whereas the major effect of the virus is on mature peripheral T cells, HIV can also infect developing thymocytes in the thymus, most of which express CD4.
Autoimmune disease
Autoimmune diseases are caused by a hyperactive immune system that instead of attacking pathogens reacts against the host organism (self) causing disease. One of the primary functions of the thymus is to prevent autoimmunity through the process of central tolerance, immunologic tolerance to self antigens.
APECED
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) is an extremely rare genetic autoimmune syndrome. However, this disease highlights the importance of the thymus in prevention of autoimmunity. This disease is caused by mutations in the Autoimmune Regulator (AIRE) gene.[13] AIRE allows for the ectopic expression of tissue-specific proteins in the thymus medulla, such as proteins that would normally only be expressed in the eye or pancreas. This expression in the thymus, allows for the deletion of autoreactive thymocytes by exposing them to self-antigens during their development, a mechanism of central tolerance. Patients with APECED develop an autoimmune disease that affects multiple endocrine tissues.
Thymoma-associated multiorgan autoimmunity (TAMA)
A GVHD-like disease called thymoma-associated multiorgan autoimmunity (TAMA) can occur in patients with thymoma. In these patients rather than a donor being a source of pathogenic T cells, the patient's own malignant thymus produces self-directed T cells. This is because the malignant thymus is incapable of appropriately educating developing thymocytes to eliminate self-reactive T cells. The end result is a disease virtually indistinguishable from GVHD.[14]
Myasthenia gravis
Myasthenia gravis is an autoimmune disease caused by antibodies that block acetylcholine receptors. Myasthenia gravis is often associated with thymic hyperplasia. Thymectomy may be necessary to treat the disease.
Patients with the autoimmune disease myasthenia gravis commonly (70%) are found to have thymic hyperplasia or malignancy.[15]
Cancer
Two primary forms of tumours originate in the thymus.
Thymomas
Tumours originating from the thymic epithelial cells are called thymomas, and are found in about 10-15% of patients with myasthenia gravis.[16] Symptoms are sometimes confused with bronchitis or a strong cough because the tumour presses on the recurrent laryngeal nerve. All thymomas are potentially cancerous, but they can vary a great deal. Some grow very slowly. Others grow rapidly and can spread to surrounding tissues. Treatment of thymomas often requires surgery to remove the entire thymus.
Lymphomas
Tumours originating from the thymocytes are called thymic lymphomas.[16] Lymphomas or leukemias of thymocyte origin are classified as Precursor T acute lymphoblastic leukemia/lymphoma (T-ALL).
People with an enlarged thymus, particularly children, were treated with intense radiation in the years before 1950. There is an elevated incidence of thyroid cancer and leukemia in treated individuals.[17]
Cervical thymic cyst
Cervical thymus is a rare malformation. Thymic tissue containing cysts is rarely described in the literature, ectopic glandular tissue included in the wall of cystic formation can trigger a series of problems similar to those of thymus.[18]
Thymic cysts are uncommon lesions, about 150 cases being found. While thymic cyst and ectopic cervical thymus are identified most frequently in childhood, the mean age at which thymoma is diagnosed is 45 years. However, studies have shown the existence of necrotic thymic tissue masses in the neck (asymptomatic intravital) more frequently, the incidence reaching nearly 30%. These observations may mean absence of clinical observation.[18]
Surgical removal
Thymectomy is the surgical removal of the thymus. The usual reason for a thymectomy is to gain access to the heart for surgery to correct congenital heart defects in the neonatal period. In neonates, but not older children or adults, the relative size of the thymus obstructs surgical access to the heart. Removal of the thymus in infancy results in immunodeficiency by some measures, although T cells develop compensating function and it remains unknown whether disease incidence in later life is significantly greater.[19][20][21][22] This is because sufficient T cells are generated during fetal life prior to birth. These T cells are long-lived and can proliferate by homeostatic proliferation throughout the lifetime of the patient. However, there is evidence of premature immune aging in patients thymectomized during early childhood.[23]
Other indications for thymectomy include the removal of thymomas and the treatment of myasthenia gravis. Thymectomy is not indicated for the treatment of primary thymic lymphomas. However, a thymic biopsy may be necessary to make the pathologic diagnosis.[23]
Research
Thymus transplantation
A thymus may be transplanted, however, this approach is problematic due to donor requirements and matching tissue with the patient.
Thymus tissue engineering
A fully functional thymus derived from reprogrammed mouse embryonic fibroblasts has been grown in the kidney capsule of mice. The newly formed organ resembled a normal thymus histologically and molecularly, and upon transplantation it was able to restore immune function in immunocompromised mice. The mouse embryonic fibroblasts were reprogrammed into thymic epithelial cells (TECs) by enforcing the expression of one transcription factor, FOXN1.[24][25]
Society and culture
When used as food for humans, animal thymic tissue is known as (one of the kinds of) sweetbread.
History
The thymus was known to the ancient Greeks, and its name comes from the Greek word θυμός (thumos), meaning "anger",[26] or "heart, soul, desire, life", possibly because of its location in the chest, near where emotions are subjectively felt; or else the name comes from the herb thyme (also in Greek θύμος or θυμάρι), which became the name for a "warty excrescence", possibly due to its resemblance to a bunch of thyme.[27][28]
Galen was the first to note that the size of the organ changed over the duration of a person's life.[29]
In the nineteenth century, a condition was identified as status thymicolymphaticus defined by an increase in lymphoid tissue and an enlarged thymus. It was thought to be a cause of sudden infant death syndrome but is now an obsolete term.[30][31]
Due to the large numbers of apoptotic lymphocytes, the thymus was originally dismissed as a "lymphocyte graveyard", without functional importance. The importance of the thymus in the immune system was discovered in 1961 by Jacques Miller, by surgically removing the thymus from one day old mice, and observing the subsequent deficiency in a lymphocyte population, subsequently named T cells after the organ of their origin.[32][33] Recently, advances in immunology have allowed the function of the thymus in T-cell maturation to be more fully understood.[34]
Other animals

The thymus is present in all jawed vertebrates, where it undergoes the same shrinkage with age and plays the same immunological function as in human beings. Recently, a discrete thymus-like lympho-epithelial structure, termed the thymoid, was discovered in the gills of larval lampreys.[35] Hagfish possess a protothymus associated with the pharyngeal velar muscles, which is responsible for a variety of immune responses.[36] Little is known about the immune mechanisms of tunicates[37] or of Amphioxus.
The thymus is also present in most vertebrates, with similar structure and function as the human thymus. Some animals have multiple secondary (smaller) thymi in the neck; this phenomenon has been reported for mice[38] and also occurs in 5 out of 6 human fetuses.[39] As in humans, the Guinea pig's thymus naturally atrophies as the animal reaches adulthood, but the athymic hairless guinea pig (which arose from a spontaneous laboratory mutation) possesses no thymic tissue whatsoever, and the organ cavity is replaced with cystic spaces.
Additional images
- Thymus of a fetus
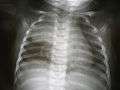 On chest X-ray, the thymus appears as a radiodense (brighter in this image) mass by the upper lobe of the child's right (left in image) lung.
On chest X-ray, the thymus appears as a radiodense (brighter in this image) mass by the upper lobe of the child's right (left in image) lung.
References
This article incorporates text in the public domain from page 1273 of the 20th edition of Gray's Anatomy (1918)
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Susan Standring; et al., eds. (2008). Gray's anatomy : the anatomical basis of clinical practice (40th ed.). London: Churchill Livingstone. ISBN 978-0-8089-2371-8.
- 1 2 3 4 5 6 7 Deakin, Barbara Young; et al. (2006). Wheater's functional histology : a text and colour atlas. Drawings by Philip J. (5th ed.). [Edinburgh?]: Churchill Livingstone/Elsevier. pp. 215–7. ISBN 9780443068508.
- ↑ Larsen, W (2001). Human Embryology (3rd ed.). Elsevier. pp. 366–367. ISBN 0-443-06583-7.
- ↑ Gray, H. (1918). "4c. The Thymus". Anatomy of the Human Body. Philadelphia: Lea & Febiger.
- ↑ Swiss embryology (from UL, UB, and UF) qblood/lymphat03
- ↑ Venturi, S; Venturi. M (2009). "Iodine, thymus, and immunity". Nutrition. 25 (9): 977–979. doi:10.1016/j.nut.2009.06.002/ (inactive 2018-06-15). PMID 19647627.
- ↑ Sutherland, J. S. (2005). "Activation of thymic regeneration in mice and humans following androgen blockade". J Immunol. 175 (4): 2741–53. doi:10.4049/jimmunol.175.4.2741. PMID 16081852.
- ↑ Hussain, I.; P.H. Win; S. Guduri (February 2, 2006). "DiGeorge Syndrome". eMedicine. Retrieved 2008-09-29.
- ↑ Miller, J. F. (2002). "The discovery of thymus function and of thymus-derived lymphocytes". Immunol Rev. 185 (1): 7–14. doi:10.1034/j.1600-065X.2002.18502.x. PMID 12190917.
- ↑ Schwarz, B. A.; Bhandoola, A. (2006). "Trafficking from the bone marrow to the thymus: a prerequisite for thymopoiesis". Immunol Rev. 209 (1): 47–57. doi:10.1111/j.0105-2896.2006.00350.x. PMID 16448533.
- ↑ Sleckman, B. P. (2005). "Lymphocyte antigen receptor gene assembly: multiple layers of regulation". Immunol Res. 32 (1–3): 253–258. doi:10.1385/IR:32:1-3:253.
- ↑ Baldwin, T. A.; Hogquist, K. A.; Jameson, S. C. (2004). "The fourth way? Harnessing aggressive tendencies in the thymus". J Immunol. 173 (11): 6515–20. doi:10.4049/jimmunol.173.11.6515. PMID 15557139.
- ↑ Peterson, P. R.; Org, T. N.; Rebane, A. (2008). "Transcriptional regulation by AIRE: Molecular mechanisms of central tolerance". Nature Reviews Immunology. 8 (12): 948–957. doi:10.1038/nri2450. PMC 2785478. PMID 19008896.
- ↑ Wadhera A, Maverakis E, Mitsiades N, Lara PN, Fung MA, Lynch PJ (Oct 2007). "Thymoma-associated multiorgan autoimmunity: a graft-versus-host-like disease". J Am Acad Dermatol. 57 (4): 683–9. doi:10.1016/j.jaad.2007.02.027. PMID 17433850.
- ↑ Kumar, Parveen J.; Clark, Michael L. (2002). Clinical Medicine (5th ed.). Saunders. p. 1222. ISBN 0-7020-2606-9.
- 1 2 Huete-Garin, A.; S.S. Sagel (2005). "Chapter 6: "Mediastinum", Thymic Neoplasm". In J.K.T. Lee; S.S. Sagel; R.J. Stanley; J.P. Heiken. Computed Body Tomography with MRI Correlation. Philadelphia: Lippincott Williams & Wilkins. pp. 311–324. ISBN 0-7817-4526-8.
- ↑ Shore, R. E.; Woodward, E.; Hildreth, N.; et al. (1985). "Thyroid Tumors Following Thymus Irradiation". J Natl Cancer Inst. 74 (6): 1177–1184. doi:10.1093/jnci/74.6.1177. PMID 3858590.
- 1 2 Octavian Dincă; Cristina Pădurariu; Alexandru Bucur (Oct 2011). "A rare entity — cervical thymic cyst". Rev. chir. oro-maxilo-fac. implantol. (in Romanian). 2 (3): 1–5. ISSN 2069-3850. 38. Retrieved 2012-06-06. (webpage has a translation button)
- ↑ Sauce, D.; et al. (2009). "Evidence of premature immune aging in patients thymectomized during early childhood". J Clin Invest. 119 (10): 3070–3078. doi:10.1172/JCI39269. PMC 2752077. PMID 19770514.
- ↑ Torfadottir, H.; Freysdottir, J.; Skaftadottir, I.; Haraldsson, A.; Sigfusson, G.; Ogmundsdottir, H. M. (2006). "Evidence for extrathymic T cell maturation after thymectomy in infancy". Clinical and Experimental Immunology. 145 (3): 407–412. doi:10.1111/j.1365-2249.2006.03139.x. PMC 1809694. PMID 16907907.
- ↑ Eysteinsdottir, J. H.; et al. (2004). "The influence of partial or total thymectomy during open heart surgery in infants on the immune function later in life". Clin Exp Immunol. 136 (2): 349–355. doi:10.1111/j.1365-2249.2004.02437.x. PMC 1809033. PMID 15086401.
- ↑ Gerli, R.; Paganelli, R.; Cossarizza, A.; Muscat, C.; Piccolo, G.; Barbieri, D.; Mariotti, S.; Monti, D.; Bistoni, O. (1999-05-01). "Long-term immunologic effects of thymectomy in patients with myasthenia gravis". The Journal of Allergy and Clinical Immunology. 103 (5 Pt 1): 865–872. doi:10.1016/S0091-6749(99)70431-8. ISSN 0091-6749. PMID 10329821.
- 1 2 Journal of Clinical Investigation. "Evidence of premature immune aging in patients thymectomized during early childhood". JCI. Retrieved 2012-06-11.
- ↑ Bredenkamp, Nicholas; Ulyanchenko, Svetlana; O’Neill, Kathy Emma; Manley, Nancy Ruth; Vaidya, Harsh Jayesh; Blackburn, Catherine Clare (2014). "An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts". Nature Cell Biology. 16 (9): 902–908. doi:10.1038/ncb3023. PMC 4153409. PMID 25150981.
- ↑ MRC, Medical Research Council, (2015-02-12). "Fully functional immune organ grown in mice from lab-created cells". www.mrc.ac.uk. Retrieved 2017-04-07.
- ↑ "Translation of Greek word "θυμός" in English". Retrieved 5 October 2012.
- ↑ θυμός, Henry George Liddell, Robert Scott, A Greek-English Lexicon, on Perseus
- ↑ Online Etymology Dictionary
- ↑ Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H (2006). "The thymus: a comprehensive review". Radiographics. 26 (2): 335–48. doi:10.1148/rg.262045213. PMID 16549602.
- ↑ "Status thymicolymphaticus".
- ↑ Sapolsky, Robert M. (2004). Why zebras don't get ulcers (3rd ed.). New York: Henry Hold and Co./Owl Books. pp. 182–185. ISBN 0805073698.
- ↑ Miller JF (2002). "The discovery of thymus function and of thymus-derived lymphocytes". Immunol. Rev. 185 (1): 7–14. doi:10.1034/j.1600-065X.2002.18502.x. PMID 12190917.
- ↑ Miller JF (2004). "Events that led to the discovery of T-cell development and function--a personal recollection". Tissue Antigens. 63 (6): 509–17. doi:10.1111/j.0001-2815.2004.00255.x. PMID 15140026.
- ↑ http://www.thymusfunctions.com
- ↑ Bajoghli; et al. (2011). "A thymus candidate in lampreys". Nature. 470 (7332): 90–94. doi:10.1038/nature09655. PMID 21293377.
- ↑ Riviere; et al. (1975). "In Search of the Hagfish Thymus" (PDF). American Zoologist. 15 (1): 39–49. doi:10.1093/icb/15.1.39. JSTOR 3882269.
- ↑ Sawada (1992). "Tunicates and Their Immune Mechanism" (PDF). Bull. Yamaguchi Med. Sch. 39 (3–4): 83–88.
- ↑ Terszowski, G; et al. (2006). "Evidence for a Functional Second Thymus in Mice". Science. 312 (5771): 284–7. doi:10.1126/science.1123497. PMID 16513945.
- ↑ Surprise organ discovered in mice, Nature News, 2 March 2006
External links
| Wikimedia Commons has media related to Thymus (organ). |
- Virtual Slidebox at Univ. Iowa Slide 287
- T cell development in the thymus. Video by Janice Yau, describing stromal signaling and tolerance. Department of Immunology and Biomedical Communications, University of Toronto. Masters Research Project, Master of Science in Biomedical Communications. 2011.