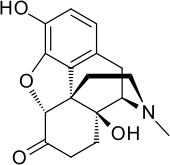
Oxymorphone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Numorphan, Numorphone, Opana, others |
| Synonyms | 14-Hydroxydihydromorphinone |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610022 |
| Pregnancy category |
|
| Dependence liability | High |
| Routes of administration | By mouth, intravenous, intramusucular, subcutaneous, rectal, intranasal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
by mouth: 10% Intranasal: 43%[2] IV, IM: 100%[3] |
| Protein binding | 10%[3] |
| Metabolism | Liver (CYP3A4, glucuronidation)[3] |
| Elimination half-life | 7–9 hours[3] |
| Excretion | Urine, feces[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.000.873 |
| Chemical and physical data | |
| Formula | C17H19NO4 |
| Molar mass | 301.337 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Oxymorphone, sold under the brand names Numorphan among others, is a powerful semi-synthetic opioid analgesic (painkiller) developed in Germany in 1914. Pain relief after injection begins after about 5–10 minutes and 15–30 minutes after rectal administration, and lasts about 3–4 hours for immediate-release tablets and 12 hours for extended-release tablets.[3]
It is highly addictive and in June 2017 the FDA asked the manufacturer to remove its product from the US market.[4] This was in part due to the opioid epidemic in the US, and the fact that the 2012 reformulation led to a shift in the route of abuse from nasal to injection. It was the first time in the history of the agency, that the FDA had made a request for removal regarding a product currently on the market. In response, by July 2017, Endo International voluntarily removed Opana ER from the market.[5] Generic versions of extended release oxymorphone are still available for prescription use in the US.
Medical uses
Oxymorphone is indicated for the relief of moderate to severe pain and also as a preoperative medication to alleviate apprehension, maintain anaesthesia and as an obstetric analgesic. It can be used to alleviate pain in dyspnea associated with acute left ventricular failure and pulmonary edema.[6] It has practically no cough suppressing activity.[6]
Oxymorphone extended-release tablets are indicated for the management of chronic pain and only for people already on a regular schedule of strong opioids for a prolonged period. Immediate-release oxymorphone tablets are recommended for breakthrough pain for people on the extended-release version. Some protocols for severe breakthrough pain in chronic pain conditions add Numorphan ampoules as a third form of the drug. As of 2009, an oxymorphone nasal spray was being developed for this purpose with unknown release date; some practitioners prefer fentanyl immediate-release formulations such as Actiq or Fentora for this purpose despite fentanyl's occasional severe side effects.[3] In the United States it is a Schedule II controlled substance with an ACSCN of 9652.
Side effects
The principal adverse effects of oxymorphone are similar to other opioids with constipation, nausea, vomiting, dizziness, dry mouth and drowsiness being the most common adverse effects. This drug is highly addictive as with other opioids and can lead to chemical dependence and withdrawal.[6]
Overdose
In common with other opioids, oxymorphone overdosage is characterized by respiratory depression, sleepiness progressing to stupor or coma, skeletal muscle weakness, cold and clammy skin, and sometimes slow heart rate and low blood pressure. In a severe case of overdose, apnea, circulatory collapse, cardiac arrest and death can occur.[6]
Pharmacology
Pharmacodynamics
Oxymorphone elicits its effects by binding to and activating the μ-opioid receptor (MOR) and, to a much lesser extent, the δ-opioid receptor (DOR) and κ-opioid receptor (KOR).[3] Its activity at the DOR may augment its action at the MOR.[3] Oxymorphone is 10 times more potent than morphine.[7]
| Affinities (Ki) | Ratio | ||
|---|---|---|---|
| MOR | DOR | KOR | MOR:DOR:KOR |
| 0.78 nM | 50 nM | 137 nM | 1:64:176 |
| Compound | Route | Dose |
|---|---|---|
| Codeine | PO | 200 mg |
| Hydrocodone | PO | 20–30 mg |
| Hydromorphone | PO | 7.5 mg |
| Hydromorphone | IV | 1.5 mg |
| Morphine | PO | 30 mg |
| Morphine | IV | 10 mg |
| Oxycodone | PO | 20 mg |
| Oxycodone | IV | 10 mg |
| Oxymorphone | PO | 10 mg |
| Oxymorphone | IV | 1 mg |
Pharmacokinetics
Chemistry
Oxymorphone is commercially produced from thebaine, which is a minor constituent of the opium poppy (Papaver somniferum) but thebaine is found in greater abundance (3%) in the roots of the oriental poppy (Papaver orientale).[3][12] German patents from the mid-1930s indicate that oxymorphone as well as hydromorphone, hydrocodone, oxycodone, and acetylmorphone can be prepared—without the need for hydrogen gas—from solutions of codeine, morphine, and dionine by refluxing an acidic aqueous solution, or the precursor drug dissolved in ethanol, in the presence of Column 7 metals, namely palladium and platinum in fine powder or colloidal form or platinum black. It is unclear if aluminium or nickel can be used as a catalyst in these reactions.[13]
Oxymorphone hydrochloride occurs as odourless white crystals or white to off-white powder. It darkens in colour with prolonged exposure to light. One gram of oxymorphone hydrochloride is soluble in 4 ml of water and it is sparingly soluble in alcohol and ether. It degrades upon contact with light.[6]
Oxymorphone can be acetylated like morphine, hydromorphone, and some other opioids. Mono-, di-, tri-, and tetra- esters of oxymorphone were developed in the 1930s but are not used in medicine at this time. Presumably other esters such as nicotinyl, benzoyl, formyl, cinnimoyl &c.can be produced.
The 2013 US DEA annual manufacturing quotas were 18 375 kilogrammes for conversion (a number of drugs can be made from oxymorphone, both painkillers and opioid antagonists like naloxone) and 6875 kg for direct manufacture of end-products.[14] Oxymorphone is also a minor metabolite of oxycodone, which is formed by CYP2D6-mediated O-demethylation.[3]
History
Oxymorphone was first developed in Germany in 1914,[15] and patented in the USA by Endo Pharmaceuticals in 1955.[16] It was introduced in the United States in January 1959 and other countries around the same time.[3]
Society and culture
Brand names
- Numorphan (suppository and injectable solution)
- Opana ER (extended-release tablet): June 2017 FDA removal request due to rates of IV abuse.[17]
- Opana IR (immediate-release tablet)
- O-Morphon in Bangladesh by Ziska pharmaceutical ltd.
The brand name Numorphan is derived by analogy to the Nucodan name for an oxycodone product (or vice versa) as well as Paramorphan/Paramorfan for dihydromorphine and Paracodin (dihydrocodeine). The only commercially available salt of oxymorphone in most of the world at this time is the hydrochloride, which has a free base conversion ratio of 0.891, and oxymorphone hydrochloride monohydrate has a factor of 0.85.[6]
Generic pill markings are ATV10/APO; HK10 (10 mgs) oblong white and ATV20/APO; HK20 (20mgs) oblong white.
Abuse and overdose
In 1924, the United States Congress had banned the sale, importation, or manufacture of heroin, another opioid pain medication in the Anti-Heroin Act of 1924.
In the United States, as of 2013 more than 12 million people abused opioid drugs at least once a year.[18] In 2010, 16,652 deaths were related to opiate overdose, in 2015 this number increased to 33,091.[19][20] In September 2013, new FDA labeling guidelines for long-acting and extended-release opioids required manufacturers to remove moderate pain as use indication, reserving the drug for "pain severe enough to require daily, around-the-clock, long-term opioid treatment"[21] however it did not restrict physicians from prescribing opioids for moderate, "as needed" usage.[18]
In January 2013, the Centers for Disease Control and Prevention reported an illness associated with intravenous (IV) abuse of oral Opana ER (oxymorphone) in Tennessee. The syndrome resembled that of thrombotic thrombocytopenic purpura (TTP).[22] Initial therapy included therapeutic plasma exchange, as for TTP. Unlike TTP, no deficient ADAMTS13 activity nor anti-ADAMTS13 antibody was found indicating a thrombotic microangiopathy of different underlying cause. If IV Opana abuse is acknowledged, supportive care, instead of therapeutic plasma exchange could be considered.[23]
In late March 2015, reports indicated Austin, Indiana, was the center of an outbreak of HIV caused by oxymorphone use as an injectable recreational drug. The outbreak required emergency action by state officials.[24][25][26] The NPR podcast "embedded" episode of March 31, 2016 was an in-depth account of a visit to oxymorphone abusers in Austin, Indiana. The current street price of oxymorphone was reported to be $140.[27]
In June 2017, faced with the public health crisis, the opioid epidemic, the FDA asked Endo Pharmaceuticals to "remove its opioid pain medication, reformulated Opana ER (oxymorphone hydrochloride), from the market". In their June 8, 2017 press release they also noted that, this was the first time the FDA had taken steps to "remove a currently marketed opioid pain medication from sale due to public health consequences of abuse."[17] By July 6, 2017, Endo International voluntarily complied with the FDA removal request.[28]
See also
References
- ↑ "Drugs@FDA: FDA Approved Drug Products". www.accessdata.fda.gov. Retrieved 7 November 2017.
- ↑ Hussain, Munir A.; Aungst, Bruce J. (1997). "Intranasal Absorption of Oxymorphone". Journal of Pharmaceutical Sciences. 86 (8): 975–6. doi:10.1021/js960513x. PMID 9269879.
- 1 2 3 4 5 6 7 8 9 10 11 12 Davis, MP; Glare, PA; Hardy, J (2009) [2005]. Opioids in Cancer Pain (2nd ed.). Oxford, UK: Oxford University Press. ISBN 978-0-19-157532-7.
- ↑ Chem. Eng. News 95(25), 8, 2017
- ↑ Commissioner, Office of the. "Press Announcements - FDA requests removal of Opana ER for risks related to abuse". www.fda.gov.
- 1 2 3 4 5 6 Brayfield, A, ed. (30 January 2013). "Oxymorphone Hydrochloride". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 5 May 2014.
- ↑ Prommer, E (February 2006). "Oxymorphone: a review". Supportive Care in Cancer. 14 (2): 109–15. doi:10.1007/s00520-005-0917-1. PMID 16317569.
- ↑ Corbett, A. D.; Paterson, S. J.; Kosterlitz, H. W. (1993). "Selectivity of Ligands for Opioid Receptors". 104 / 1: 645–679. doi:10.1007/978-3-642-77460-7_26. ISSN 0171-2004.
- ↑ King (25 October 2010). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 332–. ISBN 978-1-4496-1073-9.
- ↑ David H. Chestnut; Cynthia A Wong; Lawrence C Tsen; Warwick D Ngan Kee, Yaakov Beilin, Jill Mhyre (28 February 2014). Chestnut's Obstetric Anesthesia: Principles and Practice E-Book. Elsevier Health Sciences. pp. 611–. ISBN 978-0-323-11374-8.
- ↑ Adriana P. Tiziani (1 June 2013). Havard's Nursing Guide to Drugs. Elsevier Health Sciences. pp. 933–. ISBN 978-0-7295-8162-2.
- ↑ Corrigan, D; Martyn, EM (May 1981). "The thebaine content of ornamental poppies belonging to the papaver section oxytona". Planta Medica. 42 (1): 45–9. doi:10.1055/s-2007-971544. PMID 17401879.
- ↑ "Dihydromorphinones from Morphine and Analogs - [www.rhodium.ws]". Erowid.org. Retrieved 2012-11-03.
- ↑ "2013 - Proposed Adjustments to the Aggregate Production Quotas for Schedule I and II Controlled Substances and Assessment of Annual Needs for the List I Chemicals Ephedrine, Pseudoephedrine, and Phenylpropanolamine for 2013". www.deadiversion.usdoj.gov.
- ↑ Sinatra, Raymond (2010). The Essence of Analgesia and Analgesics. MA, USA: Cambridge University Press; 1 edition. p. 123. ISBN 978-0521144506.
- ↑ US patent 2806033, Mozes Juda Leweustein, "Morphine derivative", published 1955-03-08, issued 1957-10-09
- 1 2 "FDA requests removal of Opana ER for risks related to abuse" (Press release). Silver Spring, Maryland. U.S. Food and Drug Administration. June 8, 2017. Retrieved October 26, 2017.
Today, the U.S. Food and Drug Administration requested that Endo Pharmaceuticals remove its opioid pain medication, reformulated Opana ER (oxymorphone hydrochloride), from the market... This is the first time the agency has taken steps to remove a currently marketed opioid pain medication from sale due to the public health consequences of abuse...[FDA Commissioner Scott Gottlieb, M.D.]: "We are facing an opioid epidemic – a public health crisis, and we must take all necessary steps to reduce the scope of opioid misuse and abuse.
- 1 2 Girioin, Lisa; Haely, Melissa (11 September 2013). "FDA to require stricter labeling for pain drugs". Los Angeles Times. pp. A1 and A9.
- ↑ "Drug Overdose in the United States: Fact Sheet". Centers for Disease Control. Retrieved 12 September 2013.
- ↑ Rudd, Rose A.; Seth, Puja; David, Felicita; Scholl, Lawrence (2016). "Increases in Drug and Opioid-Involved Overdose Deaths — United States, 2010–2015". MMWR. Morbidity and Mortality Weekly Report. 65 (5051): 1445–1452. doi:10.15585/mmwr.mm655051e1. ISSN 0149-2195. PMID 28033313.
- ↑ "ER/LA Opioid Class Labeling Changes and Postmarket Requirements" (PDF). FDA. Retrieved 12 September 2013.
- ↑ Centers for Disease Control Prevention (CDC) (2013). "Thrombotic thrombocytopenic purpura (TTP)-like illness associated with intravenous Opana ER abuse--Tennessee, 2012". Morbidity and Mortality Weekly Report. 62 (1): 1–4. PMID 23302815.
- ↑ Miller, Peter John; Farland, Andrew Matthew; Knovich, Mary Ann; Batt, Katharine Marie; Owen, John (2014). "Successful treatment of intravenously abused oral Opana ER-induced thrombotic microangiopathy without plasma exchange". American Journal of Hematology. 89 (7): 695–7. doi:10.1002/ajh.23720. PMID 24668845.
- ↑ Paquette, Danielle (30 March 2015). "How an HIV outbreak hit rural Indiana — and why we should be paying attention". Washington Post. Retrieved 1 April 2015.
- ↑ Conrad, C; Bradley, H. M.; Broz, D; Buddha; Centers for Disease Control Prevention (CDC); et al. (2015). "Community Outbreak of HIV Infection Linked to Injection Drug Use of Oxymorphone, Indiana, 2015". MMWR. Morbidity and Mortality Weekly Report. 64 (16): 443–4. PMID 25928470.
- ↑ Strathdee, S. A.; Beyrer, C (2015). "Threading the Needle--How to Stop the HIV Outbreak in Rural Indiana". New England Journal of Medicine. 373 (5): 397–9. doi:10.1056/NEJMp1507252. PMID 26106947.
- ↑ McEvers, Kelly (2016-03-31). "Embedded". NPR.org.
- ↑ Palmer, Eric (July 6, 2017). "Endo caves to FDA pressure, will pull Opana ER from the market". Fierce Pharma. Retrieved October 26, 2017.