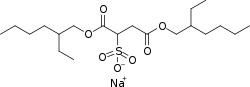
Docusate
 | |
| Clinical data | |
|---|---|
| Trade names | Colace, Ex-Lax, Senokot S |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601113 |
| Pregnancy category | |
| Routes of administration | By mouth or rectally |
| Drug class | Stool softener |
| ATC code | |
| Pharmacokinetic data | |
| Onset of action | 12 hrs to 5 days[2] |
| Duration of action | 3 days[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| E number |
E480 (thickeners, ...) |
| ECHA InfoCard |
100.008.553 |
| Chemical and physical data | |
| Formula | C20H37NaO7S |
| Molar mass | 444.56 g/mol |
| 3D model (JSmol) | |
| Density | 1.1 g/cm3 |
| Melting point | 153 to 157 °C (307 to 315 °F) |
| Solubility in water | 1 in 70 parts mg/mL (20 °C) |
| |
| |
Docusate, also known as docusate salts or dioctyl sulfosuccinate,[3] is a laxative of the stool softener type used to treat constipation.[2] It is considered a good choice in children who have hard feces.[2] For constipation that occurs as a side effect of opiate use, it may be used alone or with a stimulant laxative.[2] It may be taken as a capsule by mouth or as a rectal suppository.[2] Usually it works within one to three days.[2]
Side effects are uncommon.[2] Rarely, there may be abdominal cramps or diarrhea.[2] Efficacy decreases with long-term use, and may cause poor bowel function.[1] Docusate is acceptable during pregnancy and breastfeeding.[4] It works by allowing more water to be absorbed by the feces.[1][5] It typically comes in the form of a sodium, calcium, or potassium salt.[2]
It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[6] It is available as a generic medication and is not very expensive.[5] In the United States, one hundred doses are about 14 USD.[2] The sodium salt, dioctyl sodium sulfosuccinate, also is used as a food additive, emulsifier, dispersant, and wetting agent, among other uses.[7]
Medical use
Constipation
Docusate is used to treat constipation, and in painful anorectal conditions such as hemorrhoids and anal fissures, to help avoid pain caused by straining during bowel movements.
Given orally, the effects usually are seen 1 to 3 days after the first dose.[8] Given rectally, as an enema or suppository, a bowel movement usually occurs within 5 to 20 minutes.[9]
The drug may be used in people who are receiving opioid medication, although prolonged use may cause irritation of the gastrointestinal tract. Data supporting its efficacy in treating chronic constipation are lacking.[10]
The effectiveness of laxatives for constipation in those receiving palliative care is unclear, as it has not been sufficiently studied.[11] The comparative effectiveness of different laxatives in this group also is unclear as of 2015.[12]
Other
Docusate sodium, when used with ear syringing, may help with earwax removal, particularly in the case of impaction.[13]
Available forms
Docusate sodium may be given by mouth or rectally. It also is used as an emulsifier and dispersant in topical preparations. When taken by mouth it is typically recommended with plenty of water.
Contraindications
Docusate is contraindicated in patients with appendicitis, acute abdomen, or ileus. It is not suitable for the treatment of chronic constipation, since its mode of action is as a symptom reliever, not a cure for any specific underlying cause.[10]
Side effects
Possible side effects are typically mild and include stomach pain, diarrhea, or cramping. Serious allergic reactions may occur with the drug. The most severe side effect of docusate, although very rare, is rectal bleeding.[8]
Interactions
Docusate might increase resorption of other drugs, for example, dantron (1,8-dihydroxyanthraquinone).[10]
Other uses
Dioctyl sodium sulfosuccinate is used as a surfactant in a wide range of applications, often under the name Aerosol-OT. It is unusual in that it is able to form microemulsions without the use of co-surfactants, and it has a rich variety of aqueous-phase behavior including multiple liquid crystalline phases.[14]
- Dioctyl sodium sulfosuccinate is a pesticide adjuvant,[15] used popularly for crops of olives, almonds, wine grapes, corn, and oranges.
- It is used as an excipient in the production of tablets (as a lubricant) and suspensions (as an emulsifier).[16]
- It is the most widely used surfactant in reverse micelle encapsulation studies.[17]
Chemistry
Solubility of dioctyl sodium sulfosuccinate in water is 1:70 (14 g/l) at 25 °C, increasing to 1:20 at 70 °C. Solubility is better in less polar solvents: 1:30 in ethanol, 1:1 in chloroform and diethylether, and practically unlimited in petroleum ether (25 °C). It also is highly soluble in glycerol, although this is a rather polar solvent.
The ester groups are easily cleaved under basic conditions, but are stable against acids.[10]
Docusate salts include docusate calcium, docusate sodium, and docusate potassium.[1][3]
Mechanism of action
Docusate does not stay in the gastrointestinal tract, but is absorbed into the bloodstream and excreted via the gallbladder[10] after undergoing extensive metabolism.
The effect of docusate may not necessarily be all due to its surfactant properties. Perfusion studies suggest that docusate inhibits fluid absorption or stimulates secretion in the portion of the small intestine known as the jejunum.
Toxicity
Toxicity for different species varies widely, but dioctyl sulfosuccinate biodegrades quickly in soil and water, a typical finding being >90% in 12 to 17 days. In the atmosphere, it is predicted to be removed by a photochemical reaction with an estimated half-life of 18 hours.[18]
Humans
Dioctyl sodium sulfosuccinate is a strong irritant for eyes and lungs, and also a skin irritant. Ingestion may cause the side effects described above, such as diarrhea, intestinal bloating, and occasionally cramping pains. Dioctyl sodium sulfosuccinate is not known to be carcinogenic, mutagenic, or teratogenic.[19]
Marine species
Dioctyl sodium sulfosuccinate has been determined to be of low toxicity for crustaceans such as the hermit crab Clibanarius erythropus and the shrimp Crangon crangon. The median lethal dose (LD50) for these species is about 100 mg/l of a docusate-containing formulation after 48 hours of exposition, although the concentration of the formulation is not specified in the study.
Toxicity for molluscs varies widely, with 48-hour LD50 found between 5 mg/l for the common limpet and 100 mg/l for the common periwinkle. Various species of phytoplankton have an LD50 around 8 mg/l. All of these doses refer to a particular formulation (mentioned in the reference), not the pure chemical.[20]
In a 2010 study, dioctyl sodium sulfosuccinate exhibited higher toxicity against bacteria (Vibrio fischeri, Anabaena sp.) and algae (Pseudokirchneriella subcapitata) than did a number of fluorinated surfactants (PFOS, PFOA, or PFBS). Measuring bioluminescence inhibition of the bacteria and growth inhibition of the algae, the ED50 were in the range of 43–75 mg/l. Combinations of the fluorinated compounds with dioctyl sodium sulfosuccinate showed mid to highly synergistic effects in most settings, meaning that such combinations are significantly more toxic than the individual substances.[21]
Freshwater species
The substance is highly toxic for rainbow trout with a median lethal concentration (LC50) of 0.56 mg/l after 48 hours for the pure substance. It is only slightly to moderately toxic for rainbow trout fingerlings, and slightly toxic for harlequin rasboras (LC50 27 mg/l of a 60% formulation after 48 hours).[20]
Society and culture
Brandnames
In the U.S., dioctyl sodium sulfosuccinate is available under multiple brand names: Aqualax, Calube, Colace, Colace Micro-Enema, Correctol Softgel Extra Gentle, DC-240, Dialose, Diocto, Dioctocal, Dioctosoftez, Dioctyn, Dionex, Doc-Q-Lace, Docu Soft, Docucal, Doculax, Docusoft S, DOK, DOS, Doss-Relief, DSS, Dulcolax - Stool Softener (not to be confused with another drug marketed under the Dulcolax brand, bisacodyl, which is a stimulant laxative), Ex-Lax Stool Softener, Fleet Sof-Lax, Genasoft, Kasof, Laxa-basic, Modane Soft, Octycine-100, Pedia-Lax, Preferred Plus Pharmacy Stool Softener, Regulax SS, Sulfalax Calcium, Sur-Q-Lax, Surfak Stool Softener, and Therevac-SB. Generic preparations are also available.
In the UK, dioctyl sodium sulfosuccinate is sold under the brand name Docusol (Typharm Ltd) and DulcoEase (Boehringer Ingelheim).
In Australia, dioctyl sodium sulfosuccinate is sold as Coloxyl and Coloxyl with senna.
In India, preparations include Laxatin by Alembic, Doslax by Raptakos Laboratories, Cellubril by AstraZeneca, and Laxicon by Stadmed.
References
- 1 2 3 4 2013 Nurse's Drug Handbook. Burlington, MA: Jones & Bartlett Learning. 2013. p. 366. ISBN 9781449642846.
- 1 2 3 4 5 6 7 8 9 10 11 "Docusate Salts". The American Society of Health-System Pharmacists. Archived from the original on 2015-09-23. Retrieved Aug 11, 2015.
- 1 2 American Society of Health-System Pharmacists (2011-08-15). "Stool Softeners". Archived from the original on 2015-09-05.
- ↑ Yaffe, Sumner J. (2011). Drugs in pregnancy and lactation : a reference guide to fetal and neonatal risk (9 ed.). Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins. p. 1651. ISBN 9781608317080.
- 1 2 Hamilton, Richard J. (2013). Tarascon pocket pharmacopoeia : 2013 classic shirt-pocket edition (27 ed.). Burlington, Ma.: Jones & Bartlett Learning. p. 112. ISBN 9781449665869.
- ↑ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived (PDF) from the original on 13 December 2016. Retrieved 8 December 2016.
- ↑ Michael, fcompiled by; Ash, Irene (2004). Handbook of preservatives. Endicott, N.Y.: Synapse information resources. p. 375. ISBN 9781890595661.
- 1 2 drugs.com: Docusate Archived 2010-07-16 at the Wayback Machine.
- ↑ nursingTimes.com: Docusate sodium Archived 2011-07-21 at the Wayback Machine.
- 1 2 3 4 5 Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). 2 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ↑ Canadian Agency for Drugs and Technologies in Health (Jun 26, 2014). "Dioctyl Sulfosuccinate or Docusate (Calcium or Sodium) for the Prevention or Management of Constipation: A Review of the Clinical Effectiveness". PMID 25520993.
- ↑ Candy, B; Jones, L; Larkin, PJ; Vickerstaff, V; Tookman, A; Stone, P (13 May 2015). "Laxatives for the management of constipation in people receiving palliative care". The Cochrane Database of Systematic Reviews. 5: CD003448. doi:10.1002/14651858.CD003448.pub4. PMID 25967924.
- ↑ GlobalRPH.com: How effective is docusate as a cerumenolytic agent? Archived 2010-11-23 at the Wayback Machine.
- ↑ Nave, Sandrine; Eastoe, Julian; Penfold, Jeff (November 2000). "What Is So Special about Aerosol-OT? 1. Aqueous Systems". Langmuir. 16 (23): 8733–8740. doi:10.1021/la000341q.
- ↑ PAN Pesticides Database: Dioctyl sodium sulfosuccinate Archived 2010-09-05 at the Wayback Machine.
- ↑ Jasek, W, ed. (2008). Austria-Codex Stoffliste (in German) (41 ed.). Vienna: Österreichischer Apothekerverlag. p. 316. ISBN 978-3-85200-190-6.
- ↑ Flynn, P.F. (2004). "Multidimensional multinuclear solution NMR studies of encapsulated macromolecules". Prog. Nucl. Magn. Reson. Spectrosc. 45: 31–51. doi:10.1016/j.pnmrs.2004.04.003.
- ↑ Hazardous Substances Data Bank: Bis(2-Ethylhexyl) Sodium Sulfosuccinate Archived 2017-09-08 at the Wayback Machine.
- ↑ ScienceLab.com: Docusate sodium Material Safety Data Sheet Archived 2006-10-17 at the Wayback Machine.
- 1 2 PAN Pesticides Database – Chemical Toxicity Studies on Aquatic Organisms: Dioctyl sodium sulfosuccinate
- ↑ Rosal, R; Rodea-Palomares, I; Boltes, K; Fernández-Piñas, F; Leganés, F; Petre, A (2010). "Ecotoxicological assessment of surfactants in the aquatic environment: combined toxicity of docusate sodium with chlorinated pollutants". Chemosphere. 81 (2): 288–93. doi:10.1016/j.chemosphere.2010.05.050. PMID 20579683.
External links
- Stool Softeners at the N.I.H. PubMed Health resource.