Pharmacology of estradiol
| Clinical data | |
|---|---|
| Routes of administration |
• By mouth (tablet) • Sublingual (tablet) • Intranasal (nasal spray) • Transdermal (patch, gel, cream, emulsion, spray) • Vaginal (tablet, cream, insert (suppository), ring) • IM injection (oil solution) • SC injection (aq. soln.) • Subcutaneous implant |
| Drug class | Estrogen; Antigonadotropin |
| Pharmacokinetic data | |
| Bioavailability |
Oral: <5%[1] IM: 100%[2] |
| Protein binding |
~98%:[1][3] • Albumin: 60% • SHBG: 38% • Free: 2% |
| Metabolism | Liver (via hydroxylation, sulfation, glucuronidation) |
| Metabolites |
Major (90%):[1] • Estrone • Estrone sulfate • Estrone glucuronide • Estradiol glucuronide |
| Elimination half-life |
Oral: 13–20 hours[1] Sublingual: 8–18 hours[4] Transdermal (gel): 37 hours[5] IM (as EV): 4–5 days[2] IM (as EC): 8–10 days[6] IV (as E2): 1–2 hours[2] |
| Excretion |
Urine: 54%[1] Feces: 6%[1] |
The pharmacology of estradiol, an estrogen medication and naturally occurring steroid hormone, concerns its pharmacodynamics, pharmacokinetics, and various routes of administration.[7][8][9]
Estradiol is a naturally occurring and bioidentical estrogen, or an agonist of the estrogen receptor, the biological target of estrogens like endogenous estradiol.[7] Due to its estrogenic activity, estradiol has antigonadotropic effects and can inhibit fertility and suppress sex hormone production in both women and men.[10][11] Estradiol differs from non-bioidentical estrogens like conjugated estrogens and ethinylestradiol in various ways, with implications for tolerability and safety.[7]
Estradiol can be taken by mouth, held under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes.[7]
Pharmacodynamics
Mechanism of action
| Estrogen | ERα (%) | ERβ (%) | ERα (%) | ERβ (%) |
|---|---|---|---|---|
| Estradiol | 100 | 100 | 100 | 100 |
| Estrone | 4.0 | 3.5 | 2.6 | 4.3 |
| Estriol | 11.3 | 17.6 | 10.6 | 16.6 |
| Ethinylestradiol | 233 | 37.8 | 213 | 27.2 |
Estradiol is an estrogen, or an agonist of the nuclear estrogen receptors (ERs), the estrogen receptor alpha (ERα) and the estrogen receptor beta (ERβ).[7][8][12] According to one study, the EC50 value of estradiol for the human ERα is 50 pM (0.05 nM) and for the human ERβ is 200 pM (0.2 nM).[8][13] Estradiol is also an agonist of the membrane estrogen receptors (mERs), including the G protein-coupled estrogen receptor (GPER), Gq-coupled membrane estrogen receptor (Gq-mER), ER-X, and ERx.[14][15] It is far more potent as an estrogen than are other natural and bioidentical estrogens like estrone and estriol.[7]
Estradiol has little to no affinity for other steroid hormone receptors, including the androgen, progesterone, glucocorticoid, and mineralocorticoid receptors.[16][17][18] It has weak affinity for the androgen receptor, with about 8% of relative binding affinity of testosterone according to one study,[19] and shows agonistic activity at this receptor.[20] However, estrogens circulate in the picomolar range while androgens circulate in the nanomolar to micromolar range,[21][22] and in accordance with this, estradiol is active as an estrogen in target tissues at approximately 1,000-fold lower concentrations than is testosterone.[23] In addition, while estradiol did show activation of the androgen receptor in vitro at very high concentrations, its efficacy as an androgen receptor agonist was of such low potency that it was not possible to calculate an EC50 value for the activity.[20] As such, the weak activity of estradiol at the androgen receptor is unlikely to be of biological significance at normal physiological concentrations.[19][20]
The affinities of estradiol for the ERs are high (around 0.1 nM), and there is a relatively low quantity of about 10,000 to 20,000 ERs in the cytoplasm per cell in estrogen target tissues.[24] Estradiol stays bound to the ERs for about 24 hours, which is longer than that of other estrogens such as estriol (6 hours).[7] A prolonged duration of binding to the ERs (e.g., 9 to 12 hours for endometrial effects), as with estradiol, is necessary for full estrogenic responses in various tissues.[7] The ERs downregulate with exposure to estradiol, and in accordance, the expression of the ERs is dependent on estradiol concentrations.[25][26] Constant levels of estradiol may result in downregulation of the ERs and relatively diminished responses to estradiol, although this has not been assessed clinically.[25] Once bound to estradiol, the ERs are ubiquitinated and degraded by proteasomes, which is involved in ER downregulation.[26] The unbound ERα has an intracellular half-life of up to 5 days, but this shortens to 3 to 4 hours once bound to a ligand such as estradiol.[27][26] Estrogen deprivation can easily increase sensitivity to estrogens like estradiol by 10,000-fold or more, demonstrating a profound capacity of the ERs for upregulation and downregulation.[28] This increase in sensitivity is mediated by a 100-fold increase in ERs, as well as other mechanisms such as changes in coactivator sensitivity and degree of phosphorylation of transactivation factors.[28] Progestogens like progesterone and androgens like testosterone downregulate the ERs in certain tissues such as the endometrium and breasts, among others.[29][7][30]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | ||
|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7–12 | <0.1 | ||
| Estrone | <1 | <1 | 35 | <1 | <1 | 2.7 | <0.1 | ||
| Estriol | <1 | <1 | 15 | <1 | <1 | <0.1 | <0.1 | ||
| Ethinylestradiol | 15–25 | 1–3 | 112 | 1–3 | <1 | 0.18 | <0.1 | ||
| Values are percentages (%). Reference ligands (100%) were progesterone for the PR, testosterone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources:[19][16][17][18][31][32] | |||||||||
Effects in the body and brain
The ERs are expressed widely throughout the body, including in the breasts, uterus, vagina, prostate gland, fat, skin, bone, liver, pituitary gland, hypothalamus, and elsewhere throughout the brain.[33] Through activation of the ERs (as well as the mERs), estradiol has many effects, including the following:
- Promotes growth, function, and maintenance of the breasts, uterus, and vagina during puberty and thereafter[33][34]
- Mediates deposition of subcutaneous fat in a feminine pattern, especially in the breasts, hips, buttocks, and thighs[35]
- Maintains skin health, integrity, appearance, and hydration and slows the rate of aging of the skin[36]
- Produces the growth spurt and epiphyseal closure in both sexes during puberty, mediates widening of the hips in females during puberty, and maintains bone mineral density in both sexes throughout life[37][38]
- Modulates hepatic protein synthesis, such as the production of sex hormone-binding globulin (SHBG) and numerous other proteins, with consequent effects on the cardiovascular system and various other systems[9]
- Exerts negative feedback on the hypothalamic–pituitary–gonadal axis (HPG axis) by suppressing the secretion of the gonadotropins FSH and LH from the pituitary gland, thereby inhibiting gonadal sex hormone production as well as ovulation and fertility[39][9][40]
- Regulates the vasomotor system and body temperature via the hypothalamus, thereby preventing hot flashes[41][42]
- Modulates brain function, with effects on mood, emotionality, and sexuality, as well as cognition and memory[43]
- Influences the risk and/or progression of hormone-sensitive cancers including breast cancer, prostate cancer, and endometrial cancer[44][9]
Estrogen has also been found to increase the secretion of oxytocin and to increase the expression of its receptor, the oxytocin receptor, in the brain.[22] In women, a single dose of estradiol has been found to be sufficient to increase circulating oxytocin concentrations.[45]
Antigonadotropic effects
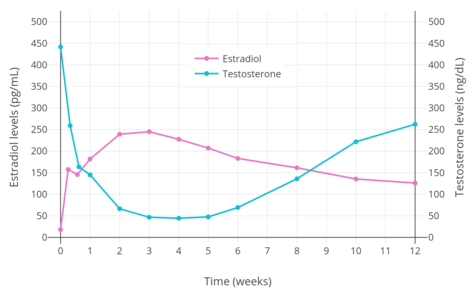
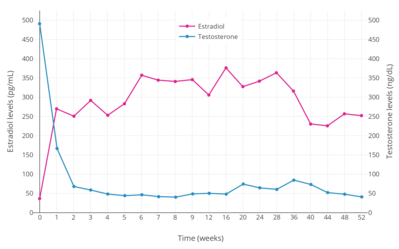
Estrogens are powerful antigonadotropins at sufficiently high concentrations.[40][51][52][10][11] By exerting negative feedback on the hypothalamic–pituitary–gonadal axis (HPG axis), they are able to suppress the secretion of the gonadotropins, LH and FSH, and thereby inhibit gonadal sex hormone production and circulating sex hormone levels as well as fertility (ovulation in women and spermatogenesis in men).[40][51][52] Clinical studies have found that in men treated with them, estrogens can maximally suppress testosterone levels by about 95% or well into the castrate/female range (< 50 ng/dL).[10][11] This is equivalent to the reduction in testosterone levels achieved by orchiectomy and gonadotropin-releasing hormone analogue (GnRH analogue) therapy, corresponding to a complete shutdown of gonadal testosterone production.[53][54] In addition, it is greater than that achieved with high-dose progestogens like cyproterone acetate and gestonorone caproate, which can maximally suppress testosterone levels in men by about 75%.[55][56][49][57][58]
Suppression of testosterone levels by estradiol to within the castrate/female range (< 50 ng/dL) in men requires relatively high levels of estradiol and has been associated with circulating levels of 200 to 300 pg/mL and above.[10][11] However, although the castrate range in men has been defined as testosterone concentrations of less than 50 ng/dL, mean levels of testosterone with surgical castration are actually about 15 ng/dL.[59] To achieve such levels of testosterone with estradiol therapy, higher concentrations of estradiol of about 500 pg/mL have been necessary to produce the requisite maximal suppression of testosterone production.[10] Injected estradiol esters like polyestradiol phosphate, estradiol valerate, and estradiol undecylate, as well as high-dose estradiol transdermal patches, are used as a form of high-dose estrogen therapy to suppress testosterone levels into the castrate range in men with prostate cancer.[9][60][61][62][11][49] High dosages of estradiol in various forms and routes are also been used to suppress testosterone levels in transgender women.[63][64][65]
Lower dosages and concentrations of estradiol can also significantly suppress gonadotropin secretion and testosterone levels in men and transgender women.[66][67] A retrospective study of oral estradiol monotherapy in transgender women found that dosages of 1 to 8 mg/day increased mean estradiol levels to about 50 to 150 pg/mL and suppressed mean testosterone levels to about 120 to 10 ng/dL.[50] However, there was high interindividual variability in the estradiol and testosterone levels achieved, and testosterone levels were insufficiently suppressed in many even at 8 mg/day.[50] In another study, a dosage of 1 mg/day oral micronized estradiol in healthy older men, which increased circulating estradiol levels by a relatively high amount of 6-fold (to 159 pg/mL), estrone levels by 15-fold (to 386 pg/mL), and SHBG levels by 17%, was found to suppress total testosterone levels by 27% (to 436 ng/dL) and free testosterone levels by 34% (to 11.8 ng/dL).[66][67]
Generally, estrogens are antigonadotropic and inhibit gonadotropin secretion.[68][69] However, in women, a sharp increase in estradiol levels to about 200 to 500 pg/mL occurs at the end of the follicular phase (mid-cycle) during the normal menstrual cycle and paradoxically triggers a surge in LH and FSH secretion.[68][70][69] During the mid-cycle surge, LH levels increase by 3- to 12-fold and FSH levels increase by 2- to 4-fold.[71][72][73] The surge lasts about 24 to 36 hours and triggers ovulation, the rupture of the dominant ovarian follicle and the release of the egg from the ovary into the oviduct.[68] This estrogen-mediated gonadotropin surge effect has also been found to occur with exogenous estrogen, including in post-hormone therapy transgender women and pre-hormone therapy transgender men acutely challenged with a high dose of an estrogen, but does not occur in men, pre-hormone therapy transgender women, or post-hormone therapy transgender men, hence indicating a hormonally-based sex difference.[74] A sufficient amount of progesterone (corresponding to levels greater than 2 ng/mL) or a progestin prevents the mid-cycle estradiol-induced surge in gonadotropin levels in women.[75][76] This is how progestins prevent ovulation and primarily mediate their contraceptive effects in women.[76]
Effects on adrenal androgen levels
In addition to their antigonadotropic effects, estrogens at high concentrations can significantly decrease androgen production by the adrenal glands.[9][77][78] A study found that treatment with a high dosage of 100 µg/day ethinylestradiol reduced circulating adrenal androgen levels by 27 to 48% in transgender women.[9][77][78] Another study found similar effects in men with prostate cancer, with levels of the adrenal androgens dehydroepiandrosterone (DHEA), dehydroepiandrosterone sulfate (DHEA-S), and androstenedione (A4) all decreasing significantly more with high-dose estrogen therapy (oral ethinylestradiol plus intramuscular polyestradiol phosphate) than with orchiectomy (by 33–39% and 10–26%, respectively).[79]
However, a study found that these effects occurred with high-dose potent oral and synthetic estrogens such as ethinylestradiol and estramustine phosphate but not with the parenteral estrogen polyestradiol phosphate, suggesting that decreases in adrenal androgen levels are secondary to changes in liver protein synthesis rather than due to a direct action in the adrenal cortex, and that such changes will only occur in the context of strong hepatic impact.[80][81] Cortisol levels were unchanged in the other groups (e.g., orchiectomy, GnRH agonist therapy, and parenteral estrogen therapy) in this study, but increased by 300 to 400% in the oral and synthetic estrogen groups, likely secondary to increases in hepatic corticosteroid-binding globulin (CBG) production and compensatory upregulation of adrenal corticosteroid synthesis.[81]
Effects on liver protein synthesis
Estradiol and other estrogens modulate liver protein synthesis via activation of hepatic ERs.[7] Estradiol increases the production and by extension circulating levels of sex hormone-binding globulin, corticosteroid-binding globulin (SHBG), angiotensinogen (AGT), pregnancy zone protein (PZP), coagulation factors, and numerous other hepatic proteins.[7] Conversely, estradiol decreases hepatic synthesis and by extension circulating levels of insulin-like growth factor 1 (IGF-1).[7] The effects of estradiol on liver protein synthesis are moderated by route of administration, with oral administration having 4- to 5-fold stronger effects on liver protein synthesis than doses by the transdermal route with equivalent general/systemic estrogenic potency.[7] The influences of estradiol on liver protein synthesis have a variety of effects in the body, with implications for the bioavailability of androgens and the cardiovascular system.[7]
| Protein | Effect |
|---|---|
| α1-Antitrypsin | + |
| Albumin | − |
| Alkaline phosphatase | + |
| Angiotensinogen | + |
| Bilirubin | + |
| Ceruloplasmin | + |
| Corticosteroid-binding globulin (transcortin) | + |
| χ-Glutamyl transpeptidase | + |
| Growth hormone | + |
| Growth hormone-binding protein | + |
| Insulin-like growth factor 1 | − |
| Haptoglobin | − |
| Leucyl aminopeptidase | + |
| α2-Microglobulin | + |
| Orosomucoid (α1-acid glycoprotein) | − |
| Pregnancy zone protein | + |
| Retinol-binding protein | + |
| Sex hormone-binding globulin | + |
| Thyroxine-binding globulin | + |
| Transferrin | + |
| Coagulation factors | Effect |
| Antithrombin III | − |
| C-reactive protein | + |
| Coagulation factor II | + |
| Coagulation factor VII | + |
| Coagulation factor VIII | + |
| Coagulation factor IX | + |
| Coagulation factor X | + |
| Coagulation factor XII | + |
| Fibrinogen | + |
| Plasminogen | + |
| Protein C | + |
| Prothrombin time | − |
| Lipids | Effect |
| Apolipoprotein A | + |
| High-density lipoprotein | + |
| Low-density lipoprotein | − |
| Lecithin | + |
| Total lipids | + |
| Triglycerides | + |
Differences from other estrogens
Estradiol has relatively low oral bioavailability of about 5%.[7] In addition, there is considerable interindividual variability in levels of estradiol achieved with oral estradiol.[7] In contrast to estradiol, the synthetic estrogen ethinylestradiol has about 45% oral bioavailability, around 80- to 200-fold greater systemic oral estrogenic potency, roughly 500- to 1,500-fold greater hepatic oral estrogenic potency, and less interindividual variability in circulating estrogen levels achieved.[54][7][1][83][84][85][86] An oral dose of ethinylestradiol that is approximately 100-fold lower than that of estradiol achieves similar maximal circulating estrogen concentrations (e.g., 50 pg/mL ethinylestradiol with a single 20 μg dose of ethinylestradiol relative to 40 pg/mL estradiol with a single 2 mg dose of micronized estradiol or estradiol valerate).[7] These differences are due to the introduction of an ethynyl group at the C17α position in ethinylestradiol (also known as 17α-ethynylestradiol), which results in steric hindrance and greatly diminishes the first-pass metabolism of ethinylestradiol relative to estradiol with oral administration.[7] Estradiol and ethinylestradiol have similar affinities for and efficacies as agonists of the ERs,[7][8] and the systemic estrogenic potency of estradiol and ethinylestradiol is similar when they are administered by the intravenous route.[3]
Synthetic estrogens like ethinylestradiol and diethylstilbestrol and the natural but animal-derived conjugated estrogens have disproportionate effects on liver protein synthesis relative to their effects in other tissues when compared to estradiol.[7] At doses via the oral route with comparable systemic estrogenic potency, conjugated estrogens have about 1.3 to 4.5 times the hepatotropic potency (i.e., potency in modulating liver protein synthesis) of estradiol, ethinylestradiol has about 2.9 to 5.0 times the hepatotropic potency of estradiol, and diethylstilbestrol shows about 5.7 to 7.5 times the hepatotropic potency of estradiol (all measured via a small selection of estrogen-modulated hepatic proteins that included HDL cholesterol, SHBG, CBG, and angiotensinogen).[7] The greater hepatotropic potency of these estrogens relative to estradiol is related to susceptibility to hepatic metabolism.[7] Whereas estradiol is metabolized and thereby inactivated rapidly upon entry into the liver, other estrogens like ethinylestradiol and diethylstilbestrol are resistant to hepatic metabolism and persist in the liver for a longer amount of time.[7] This is reflected in the biological half-lives of these estrogens; the blood half-life of estradiol is about 1 to 2 hours, while the half-lives of ethinylestradiol and diethylstilbestrol are approximately 20 hours and 24 hours, respectively.[2][87][86] In accordance with its long half-life, ethinylestradiol passes through the liver many times prior to its elimination.[88] Because humans are not adapted to efficiently metabolize conjugated estrogens (which are equine (horse) estrogens) and synthetic estrogens like ethinylestradiol and diethylstilbestrol, these estrogens are not properly inactivated in the liver, with markedly disproportionate hepatic estrogenic effects resulting.[7]
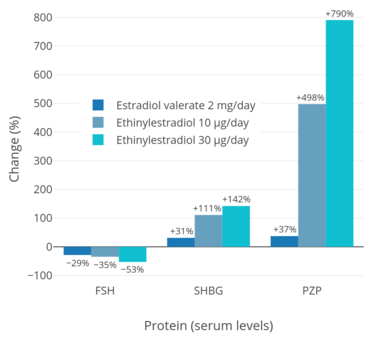
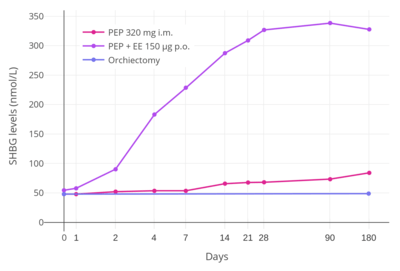
In addition to differences in hepatotropic potency between estradiol and other estrogens, there are differences in hepatotropic potency between different routes of administration of estradiol.[7] Due to the first pass through the liver, oral estradiol results in disproportionate and unphysiological hepatic estradiol levels that are 4- to 5-fold higher than in the circulation.[89][7] Conversely, parenteral routes of estradiol, such as transdermal, vaginal, and injection, bypass the first pass through the liver and produce levels of estradiol in the circulation and liver that are comparable.[89][7] As an example of the reduced hepatic impact of parenteral estradiol relative to oral estradiol, a study found that 1 mg/day oral estradiol significantly increased SHBG levels by 45%, while 50 µg/day transdermal estradiol increased SHBG levels non-significantly by only 12% (with these dosages being roughly equivalent in systemic estrogenic potency).[90][91][92] As such, not only do oral non-bioidentical estrogens like ethinylestradiol and conjugated estrogens have substantially greater potency in the liver than does oral estradiol, oral estradiol has considerably greater potency in the liver than does parenteral estradiol.[7] Thus, the hepatotropic effects of oral non-bioidentical estrogens like ethinylestradiol are massive in comparison to parenteral estradiol (see the graph above/to the right), which in contrast to these estrogens has very weak or even absent effects on liver protein synthesis at normal therapeutic dosages.[7][46][54][9] Whereas high-dosage 320 mg/month intramuscular polyestradiol phosphate increased SHBG levels to 166% in men with prostate cancer, the combination of 80 mg/month intramuscular polyestradiol phosphate and high-dosage 150 µg/day oral ethinylestradiol increased levels of SHBG to 617%, an almost 8-fold difference in increase and almost 4-fold difference in absolute levels between the two treatment regimens.[10][46][93]
The effects of estrogens on liver protein synthesis, such as on the synthesis of coagulation factors, lipoproteins, and triglycerides, can cause an increased risk of thromboembolic and cardiovascular complications, which in turn can result in increased mortality.[54] The risk of thromboembolic and cardiovascular complications is significantly increased in postmenopausal women taking oral conjugated estrogens as a component of menopausal hormone therapy.[7][94][95] Both oral estradiol and oral esterified estrogens have been found to have a significantly lower risk of thromboembolic and cardiovascular complications than oral conjugated estrogens, and transdermal estradiol appears to have no such risks at all.[7][96][94][95] Widely employed in the past, oral synthetic estrogens like ethinylestradiol and diethylstilbestrol are no longer used in menopausal hormone therapy due to their high risks of thromboembolic and cardiovascular complications.[97] Studies have found a markedly increased 5-year risk of cardiovascular mortality of 14 to 26% in men treated with high-dosage oral synthetic estrogens like ethinylestradiol and diethylstilbestrol for prostate cancer.[54] With diethylstilbestrol, there is an up to 35% incidence of cardiovascular toxicity and an up to 15% incidence of venous thromboembolism.[98] In a small study comparing high-dosage 320 mg/month intramuscular polyestradiol phosphate versus the combination of 80 mg/month polyestradiol phosphate with high-dosage 150 µg/day oral ethinylestradiol for prostate cancer, there was a 25% incidence of cardiovascular complications over the course of a year in the group that was also treated with ethinylestradiol, whereas there were no cardiovascular complications in the polyestradiol phosphate-only group.[46] In accordance, another study found no change in levels of coagulation factor VII, a protein of particular importance in the cardiovascular side effects of estrogens, with 240 mg/month intramuscular polyestradiol phosphate.[99] In spite of the markedly reduced impact of parenteral estradiol on the liver compared to other estrogens however, high dosages of parenteral estradiol, producing high levels of circulating estradiol, can still result in important and undesirable changes in liver protein synthesis as with other estrogens.[40] A high dosage of 320 mg/month polyestradiol phosphate has been found to result in significantly increased cardiovascular morbidity (due to non-fatal ischemic heart events and heart decompensation) in men with prostate cancer in two large studies, though cardiovascular mortality was notably not increased.[40][100]
In addition to the liver, ethinylestradiol shows disproportionate estrogenic effects in the uterus.[7][43][101] This is due to its inability to be inactivated by uterine 17β-hydroxysteroid dehydrogenase (17β-HSD).[7][43][101] Because of its disproportionate effects in the uterus, ethinylestradiol is associated with a significantly lower incidence of vaginal bleeding and spotting than is estradiol, particularly in combination with progestogens (which induce 17β-HSD expression and hence estradiol metabolism in the uterus),[7] and is an important contributing factor in why ethinylestradiol, among other reasons and in spite of its inferior safety profile, has been widely used in oral contraceptives instead of estradiol.[102][103] Although ethinylestradiol has increased effects in the uterus relative to estradiol, it is similarly not associated with an increase in the risk of endometrial hyperplasia and endometrial cancer when used in combination with a progestogen, but instead with a significant decrease.[7][104]
| Estrogen | Type | HF | VE | UCa | FSH | LH | HDL-C | SHBG | CBG | AGT | Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estradiol | Bioidentical | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Estrone | Bioidentical | ND | ND | ND | 0.3 | 0.3 | ND | ND | ND | ND | ND |
| Estriol | Bioidentical | 0.3 | 0.3 | 0.1 | 0.3 | ND | 0.2 | ND | ND | ND | 0.67 |
| Estrone sulfate | Bioidentical | ND | 0.9 | 0.9 | 0.9 | 0.9 | 0.5 | 0.9 | 0.7 | 1.5 | 0.56–1.7 |
| Conjugated estrogens | Natural | 1.2 | 1.5 | 2.0 | 1.1 | 1.0 | 1.5 | 3.0 | 1.5 | 5.0 | 1.3–4.5 |
| Equilin sulfate | Natural | ND | ND | ND | ND | ND | 6.0 | 7.5 | 6.0 | 7.5 | ND |
| Ethinylestradiol | Synthetic | 120 | 150 | 40 | 120 | 100 | 400 | 500 | 600 | 350 | 2.9–5.0 |
| Diethylstilbestrol | Synthetic | ND | ND | ND | 3.4 | ND | ND | 25.6 | 24.5 | 19.5 | 5.7–7.5 |
| Notes: Values are ratios, with estradiol as standard (i.e., 1.0). Abbreviations: HF = Clinical relief of hot flashes. VE = Increased proliferation of vaginal epithelium. UCa = Decrease in UCa. FSH = Suppression of FSH levels. LH = Suppression of LH levels. HDL-C, SHBG, CBG, and AGT = Increase in the serum levels of these hepatic proteins. Ratio = Ratio of liver protein effects to hot flashes relief and gonadotropin suppression. ND = No data. Type: Bioidentical = Identical to those found in humans. Natural = Naturally occurring but not identical to those found in humans (e.g., estrogens of other species). Synthetic = Man-made, does not naturally occur in animals or in the environment. Miscellaneous: Direct link to table. Sources: [7][105][106][107][108][109] | |||||||||||
Pharmacokinetics
Estradiol can be taken by a variety of different routes of administration.[7] These include oral, buccal, sublingual, intranasal, transdermal (gels, creams, patches), vaginal (tablets, creams, rings, suppositories), rectal, by intramuscular or subcutaneous injection (in oil or aqueous), and as a subcutaneous implant.[7] The pharmacokinetics of estradiol, including its bioavailability, metabolism, biological half-life, and other parameters, differ by route of administration.[7] Likewise, the potency of estradiol, and its local effects in certain tissues, most importantly the liver, differ by route of administration as well.[7] In particular, the oral route is subject to a high first-pass effect, which results in high levels of estradiol and consequent estrogenic effects in the liver and low potency due to first-pass hepatic and intestinal metabolism into metabolites like estrone and estrogen conjugates.[7] Conversely, this is not the case for parenteral (non-oral) routes, which bypass the intestines and liver.[7]
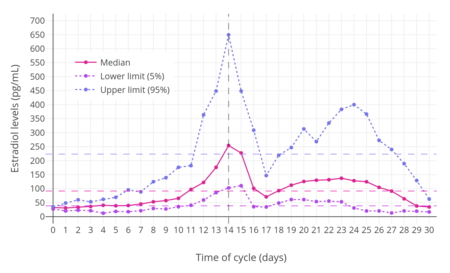
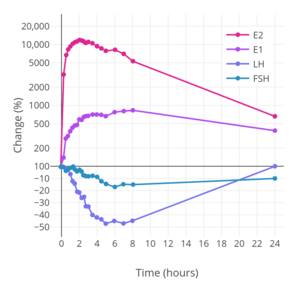
Different estradiol routes and dosages can achieve widely varying circulating estradiol levels (see the table below).[7] For purposes of comparison with normal physiological circumstances, menstrual cycle circulating levels of estradiol in premenopausal women are 40 pg/mL in the early follicular phase, 250 pg/mL at the middle of the cycle, and 100 pg/mL during the mid-luteal phase.[43] Circulating estrone levels during the menstrual cycle range from 40 to 170 pg/mL, which parallels circulating levels of estradiol.[43] Mean integrated levels of circulating estradiol in premenopausal women across the whole menstrual cycle are in the range of 80 and 150 pg/mL, according to some sources.[111][112][113] The estradiol-to-estrone ratio in premenopausal women is approximately 2:1.[7][43] In postmenopausal women, circulating levels of estradiol are below 15 pg/mL and mean levels of estrone are about 30 pg/mL; the estradiol-to-estrone ratio is reversed to approximately 1:2.[7][43] During normal human pregnancy, estrogen levels are very high.[114] Estradiol levels are 1,000 to 5,000 pg/mL during the first trimester, 5,000 to 15,000 pg/mL during the second trimester, and 10,000 to 40,000 pg/mL during the third trimester,[110] with a mean of 25,000 pg/mL at term and levels as high as 75,000 pg/mL measurable in some women.[115]
| Route | Dose | Time | E2 (↑Δ) | E1 (↑Δ) | Ratio | Ref | |||
|---|---|---|---|---|---|---|---|---|---|
| Oral | 1 mg 2 mg 4 mg | 12 hours 3 hours 6 hours | +25 pg/mL +40 pg/mL +50 pg/mL | +150 pg/mL +250 pg/mL +500 pg/mL | 0.15 0.16 0.10 | [117] [117] [117] | |||
| Sublingual | 1 mg 0.5 mg 0.5 mg 0.5 mg | 1 hour 1 hour 1 hour 1 hour | +450 pg/mL +250 pg/mL +750 pg/mL +75 pg/mL | +160 pg/mL +85 pg/mL +250 pg/mL +24 pg/mL | 3 3 3 3 | [118] [118] [119] [120] | |||
| Intranasal | 1 mg | 1 hour | +110 pg/mL | +110 pg/mL | 1.0 | [121] | |||
| Topical (gel) | 3 mg 3 mg/day 3 mg/2 days | 5 hours 12–20 hours 12 hours 36 hours | +70 pg/mL +45–279 pg/mL +300–1310 pg/mL +20–179 pg/mL | +50 pg/mL +31–230 pg/mL +24–110 pg/mL +120–500 pg/mL | 0.4 1 1 1 | [122] [123] [123] [123] | |||
| Vaginal (cream) | 0.5 mg 1.0 mg | 3 hours 3 hours | +830 pg/mL +800 pg/mL | +150 pg/mL +150 pg/mL | 5.0 5.0 | [124] [121] | |||
| Intramuscular (esters in oil) | 5 mg EB 5 mg EV 5 mg EC 100 mg EU 320 mg PEP | 1.8, 2.4 daysa 2.2, 2.7 daysa 3.9, 5.1 daysa 1 day 16, 25 daysa | 940 pg/mLb 667 pg/mLb 338 pg/mLb 500 pg/mLb 270 pg/mLb | 343 pg/mLb 324 pg/mLb 145 pg/mLb ND 1000 pg/mLb | 2.7 2.1 2.3 ND 0.27 | [125] [125] [125] [126] [46] | |||
| a = Time to peak for estradiol, estrone levels. b = Absolute levels, not increase or change in levels. | |||||||||
| Group | E2 (prod) | E2 (levels) | E1 (levels) | Ratio |
|---|---|---|---|---|
| Prepubertal boys | ND | 2–8 pg/mL | ND | ND |
| Pubertal girls Tanner stage I (childhood) Tanner stage II (ages 8–12) Tanner stage III (ages 10–13) Tanner stage IV (ages 11–14) Tanner stage V (ages 12–15) Follicular (days 1–14) Luteal (days 15–28) | ND ND ND ND ND ND | 9 (<9–20) pg/mL 15 (<9–30) pg/mL 27 (<9–60) pg/mL 55 (16–85) pg/mL 50 (30–100) pg/mL 130 (70–300) pg/mL | 13 (<9–23) pg/mL 18 (10–37) pg/mL 26 (17–58) pg/mL 36 (23–69) pg/mL 44 (30–89) pg/mL 75 (39–160) pg/mL | ND ND ND ND ND ND |
| Premenopausal women Early follicular phase (days 1–4) Mid follicular phase (days 5–9) Late follicular phase (days 10–14) Luteal phase (days 15–28) Oral contraceptive (anovulatory) | 65–100 µg/day 100–160 µg/day 320–640 µg/day 300 µg/day ND | 40–60 pg/mL 60–100 pg/mL 200–400 pg/mL 190 pg/mL 12–50 pg/mL | 40–60 pg/mL ND 170–200 pg/mL 100–150 pg/mL ND | 0.5–1 ND 1–2 1.5 ND |
| Postmenopausal women | 18 µg/day | 5–20 pg/mL | 30–70 pg/mL | 0.3–0.8 |
| Pregnant women First trimester (weeks 1–12) Second trimester (weeks 13–26) Third trimester (weeks 27–40) | ND ND ND | 1,000–5,000 pg/mL 5,000–15,000 pg/mL 10,000–40,000 pg/mL | ND ND ND | ND ND ND |
| Men | 30–60 µg/day | 25–45 pg/mL | 25–90 pg/mL | 0.4–0.6 |
| Mean levels are given as a single value and ranges are given after in parentheses. Sources:[116][127][128][114][129][110] | ||||
Oral administration
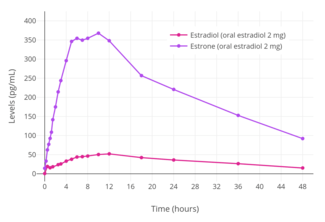
Estradiol is rapidly and completely absorbed with oral administration.[134][9] This is true for oral doses of 2 mg and 4 mg, but absorption was found to be incomplete for an oral dose of 8 mg (which showed 76% of the expected bioavailability, based on dose proportionality and area-under-the-curve levels).[134][135] The oral bioavailability of estradiol is very low, and the hormone must be either micronized or conjugated with an ester, as in estradiol valerate or estradiol acetate, to be bioavailable to an extent that is clinically useful.[136][137][138] This is because estradiol is extensively metabolized during the first pass through the intestines and liver, and micronization increases the rate of absorption and improves the metabolic stability of estradiol.[134] As micronization is required for significant bioavailability, all oral estradiol tablets are micronized.[137] The absolute bioavailability of oral micronized estradiol is approximately 5%, with a possible range of 0.1% to 12%.[1][136] In accordance, the circulating levels of estradiol with 2 mg/day oral estradiol and 100 µg/day transdermal estradiol are similar, in spite of a 20-fold difference in dosage.[139][9] There is high interindividual variability in the levels of estradiol achieved with oral estradiol, which is likely related to the high first-pass effect.[9] This variation has been reported to be in the range of 28 to 127% in terms of mean area-under-the-curve levels of estradiol.[9]
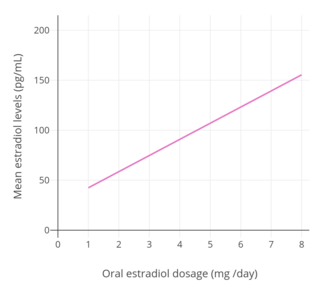
In postmenopausal women, a dosage of 1 mg/day oral micronized estradiol has been found to produce circulating concentrations of 30 to 50 pg/mL estradiol and 150 to 300 pg/mL estrone, while a dosage of 2 mg/day has been found to result in circulating levels of 50 to 180 pg/mL estradiol and 300 to 850 pg/mL estrone.[43] A study of oral micronized estradiol in transgender women found that mean estradiol levels across a dosage range of 1 to 8 mg/day were almost 50 pg/mL at 1 mg/day, almost 100 pg/mL at 4 mg/day, and just above 150 pg/mL at 8 mg/day, with a wide degree of variation.[50] A single 10 mg dose of micronized estradiol or estradiol valerate has been found to produce circulating levels of estradiol of about 250 pg/mL in postmenopausal women.[101] A study that used high- to very high-dose oral micronized estradiol to treat postmenopausal women with ER-positive breast cancer found that mean steady-state estradiol levels in the 6 mg/day group were 302 pg/mL and in the 30 mg/day group were 2,403 pg/mL.[140]
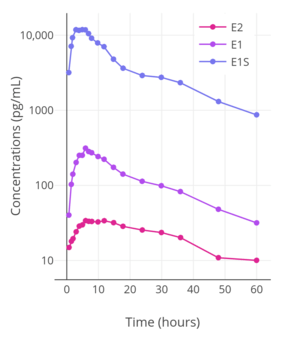
When taken orally, about 95% of a dose of estradiol is metabolized in the intestines and liver into estrone and estrogen conjugates such as estrone sulfate, estrone glucuronide, and estradiol sulfate, among others, prior to entering the circulation.[134][141][142][143] As a result, circulating estrone and estrogen conjugate levels are markedly elevated, in a highly unphysiological manner, with oral estradiol.[141][139] Whereas the ratio of circulating estradiol to estrone is about 1:1 in premenopausal women and with transdermal estradiol, oral estradiol produces a ratio of about 1:5 on average and as high as 1:20 in some women.[1][7][144][135] In addition, whereas levels of estradiol with menopausal replacement dosages of oral estradiol are in the range of the follicular phase of the normal menstrual cycle, levels of estrone resemble those during the first trimester of pregnancy.[145][146] Moreover, whereas normal estrone sulfate levels are 10 to 25 times higher than those of estradiol and estrone in premenopausal women,[147] levels of estrone sulfate with oral estradiol are an additional 10 to 20 times higher than normal premenopausal estrone sulfate levels.[139][148] One study found that estrone sulfate levels were 200-fold higher than estradiol levels with 2 mg/day oral micronized estradiol or oral estradiol valerate, and estrone sulfate levels can reach up to nearly 1,000-fold higher concentrations than estradiol in some cases.[7][9] In contrast to oral estradiol, due to the lack of the first pass, an excess in estrone and estrogen conjugate levels does not occur with transdermal estradiol or other parenteral estradiol routes.[141][139]
The transformation of estradiol into estrone and estrogen conjugates is reversible, and these metabolites hence can be converted back into estradiol.[7] About 15% of orally administered estradiol is transformed into estrone and 65% into estrone sulfate.[9] About 5% of estrone and 1.4% of estrone sulfate can be converted back into estradiol.[9] An additional 21% of estrone sulfate can be converted into estrone, whereas the transformation of estrone into estrone sulfate is approximately 54%.[9] The interconversion between estradiol and estrone is mediated by 17β-hydroxysteroid dehydrogenases (17β-HSDs),[9] whereas the conversion of estrone into estrone sulfate is mediated by estrogen sulfotransferases (ESTs) and the transformation of estrone sulfate into estrone by steroid sulfatase (STS).[149][150] The metabolic clearance rates and hence blood half-lives of estrogen conjugates like estrone sulfate are far longer than those of estradiol and estrone.[7][9][139] Estrogen conjugates, primarily estrone sulfate, serve as a large circulating reservoir for estradiol, and because of this, they function to greatly extend the biological half-life of oral estradiol.[7][9] As such, the biological half-life of oral estradiol is a composite parameter that is dependent on interconversion between estradiol and estrogen conjugates, as well as on enterohepatic recirculation.[9] Whereas the biological half-life of estradiol given by intravenous injection is only about 1 to 2 hours, the biological half-life of oral estradiol has a range of 13 to 20 hours due to the large and long-lasting pool of estrogen conjugates that is formed during first-pass metabolism and that serves to continuously replenish circulating estradiol levels.[9][7]
In contrast to estradiol, estrone has very low activity as an estrogen.[7][151][7][12] The affinities of estrone for the human ERs and its estrogenic activity have been reported to be approximately 3 to 4% of those of estradiol.[7] In addition, unlike estradiol and estriol, estrone is not accumulated in target tissues.[7] Because estrone can be transformed into estradiol, most of its activity in vivo is actually due to conversion into estradiol.[7] In accordance, dosages of oral and transdermal estradiol that achieve similar levels of estradiol have been found, in spite of markedly elevated levels of estrone with oral estradiol but not with transdermal estradiol, to possess equivalent and non-significantly different potency in terms of clinical measures including suppression of LH and FSH levels, inhibition of bone resorption, and relief of menopausal symptoms such as hot flashes.[7][141][128][152] In addition, estradiol levels were found to correlate with these effects, while estrone levels did not.[141][128] These findings suggest that estrone contributes very little or not at all to the estrogenic potency of estradiol, while also not antagonizing the estrogenic activity of estradiol.[7][141][128][152] This contradicts some in vitro research suggesting that estrone might be able to partially antagonize the actions of estradiol.[153][154][155]
On the other hand, it has been suggested that the high levels of estrone and/or estrone conjugates with oral estradiol may result in excessive estradiol levels in certain tissues such as the breasts and endometrium due to high expression of the requisite enzymes (17β-HSDs and STS) necessary to transform these metabolites back into estradiol in these tissues.[146][139][131][158] In accordance, circulating levels of estrone sulfate have been found to be positively associated with breast density in postmenopausal women treated with oral estradiol, with a 1.3% increase in breast density observed for every 1 ng/mL increase in estrone sulfate levels.[159][160] Similarly, levels of estradiol, estrone, and estrone sulfate are all strongly associated with the risk of breast cancer in women.[159] Preclinical studies have shown that estrone sulfate, via local transformation into estradiol, stimulates the growth of mammary cancer cells.[161][162] In addition, a study found that administration of estrone sulfate was more efficient in delivering free estradiol into mammary cancer cells and increasing mammary tumor volume than was administration of estradiol itself.[161]
Due to the first pass through the liver, disproportionate and supraphysiological levels of estrogens occur locally in the liver with oral estradiol.[89][9] These levels are approximately 4- to 5-fold higher than in the circulation.[89] As a result, there is abnormally high estrogenic signaling in the liver with oral estradiol, and a variety of unphysiological effects on liver protein synthesis result.[7][9] Via modulation of liver protein synthesis, oral estradiol increases the risk of blood clots,[163] suppresses growth hormone (GH)-mediated insulin-like growth factor 1 (IGF-1) production,[164][165] increases circulating levels of a variety of binding proteins including thyroid binding globulin (TBG), cortisol binding globulin (CBG), sex hormone binding globulin (SHBG), growth hormone binding protein (GHBP),[166] insulin-like growth factor-binding proteins (IGFBPs),[167] and copper binding protein (CBP),[142][168] and produces positive blood lipid changes, among a variety of other effects.[143][169][9] In contrast to oral estradiol, transdermal estradiol has minimal to no impact on liver protein synthesis.[7] As an example, a study found that 1 mg/day oral estradiol significantly increased SHBG levels by 45%, while 50 µg/day transdermal estradiol increased SHBG levels non-significantly by only 12%.[90][91][92]
In the circulation, approximately 38% of estradiol is reversibly bound to SHBG and 60% is reversibly bound to albumin in women under normal physiological circumstances, with 2 to 3% of total estradiol circulating free or unbound at any given time.[134][136][1] Only estradiol that is free or unbound is able to be enter target cells and hence is biologically active.[1][9]:249[112] The increase in SHBG levels with oral estradiol (e.g., +50%) can result in a clinically meaningful increase in the fractions of sex hormones like estradiol and testosterone that are bound to SHBG, whereas this is not the case with typical clinical dosages of transdermal estradiol.[170][112] The increase in the fraction of estradiol bound to SHBG results in a significant decrease in the percentage of free or unbound and hence bioactive estradiol.[1][112] As a result, the bioavailability and potency of oral estradiol may be diminished relative to parenteral estradiol routes by some amount.[112][1]
| Route | Treatment | Levels (pg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E2 | E2G | E2S | E1 | E1G | E1S | E3 | E3G | E3S | ||
| Oral | ||||||||||
| Before | 11 | <9 | <7 | 47 | 41 | 472 | UD | <11 | <8 | |
| 1 mg/day | 51 | 444 | 46 | 202 | 1018 | 1416 | UD | 297 | 198 | |
| 2 mg/day | 78 | 789 | 91 | 437 | 2282 | 3073 | UD | 569 | 402 | |
| Transdermal | ||||||||||
| Before | 12 | <9 | <7 | 40 | 40 | 340 | UD | <11 | <9 | |
| 100 µg/day | 90 | 70 | 20 | 82 | 170 | 831 | UD | 79 | 41 | |
Buccal administration
Estradiol has been studied for use by buccal administration.[7][171][172][173]
Sublingual administration
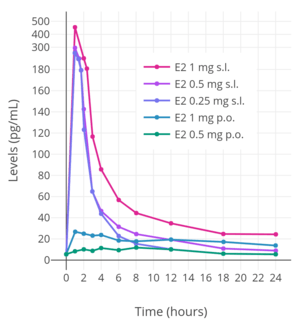

Micronized estradiol tablets can be taken sublingually instead of orally.[175] All estradiol tablets are micronized, as estradiol cannot be absorbed efficiently otherwise.[137] Sublingual ingestion bypasses first-pass metabolism in the intestines and liver.[176] It has been found to result in levels of estradiol and an estradiol-to-estrone ratio that are substantially higher in comparison to oral ingestion.[174] Maximal circulating levels of estradiol are as much as 10-fold higher with sublingual administration relative to oral administration, and the absolute bioavailability of estradiol is approximately 5-fold higher.[7] On the other hand, levels of estradiol fall rapidly with sublingual administration, whereas they remain elevated for an extended period of time with oral administration.[7][9] This is responsible for the divergence between the maximal estradiol levels achieved and the absolute bioavailability.[7][9]
The rapid and steep fall in estradiol levels with sublingual administration is analogous to the case of intravenous administration of the hormone, in which there is a rapid distribution phase of 6 minutes and terminal disposition phase of only 1 to 2 hours.[7][9][2] In contrast to intravenous and sublingual administration, the terminal half-life of estradiol with oral administration is 13 to 20 hours.[7][9] The difference is due to the fact that, upon oral administration, a large hormonally inert pool of estrogen sulfate and glucuronide conjugates with extended terminal half-lives is reversibly formed from estradiol during first-pass metabolism, and this pool serves as a metabolism-resistant and long-lasting circulating reservoir for slow reconversion back into estradiol.[7][9]
Upon sublingual ingestion, a single 0.25 mg tablet of micronized estradiol has been found to produce peak levels of 300 pg/mL estradiol and 60 pg/mL estrone within 1 hour.[7] A higher dose of 1 mg estradiol was found to result in maximum levels of 450 pg/mL estradiol and 165 pg/mL estrone.[7] This was followed by a rapid decline in estradiol levels to 85 pg/mL within 3 hours, whereas the decline in estrone levels was much slower and reached a level of 80 pg/mL after 18 hours.[7]
Although sublingual administration of estradiol has a relatively short duration, the drug can be administered multiple times per day in divided doses to compensate for this.[7] In addition, it is notable that the magnitude of the genomic effects of estradiol (i.e., signaling through the nuclear ERs) may, at least in some cases, be dependent on the total exposure as opposed to the duration of exposure.[7] For instance, in normal human epithelial breast cells and ER-positive breast cancer cells, the rate of breast cell proliferation has been found not to differ with estradiol incubation of 1 nM for 24 hours and incubation of 24 nM for 1 hour.[7] In other words, short-term high concentrations and long-term low concentrations of estradiol appear to have the same degree of effect in terms of genomic estrogenic signaling, at least in terms of breast cell proliferation.[7]
On the other hand, non-genomic actions of estradiol, such as signaling through membrane estrogen receptors like the GPER, may be reduced with short-term high concentrations of estradiol relative to more sustained levels.[7] For instance, although daily intranasal administration of estradiol (which, similarly to sublingual administration, produces extremely high peak levels of estradiol followed by a rapid fall in estradiol levels) is associated in postmenopausal women with comparable clinical effectiveness (e.g., for hot flashes) relative to longer acting routes of estradiol administration, it is also associated with significantly lower rates of breast tension (tenderness and enlargement) relative to longer acting estradiol routes, and this is thought to reflect comparatively diminished non-genomic signaling.[7]
The effects of sublingual estradiol on gonadotropin levels have been studied in postmenopausal women.[119]
Intranasal administration
Intranasal administration of estradiol has been studied.[171][7]
Estradiol is or was available as a nasal spray (brand name Aerodiol) in some countries.[177][178][179][180] The Aerodiol product was discontinued in 2007.[181][182] Intranasal estradiol has pharmacokinetics similar to those of sublingual estradiol, including a sharp peak in levels followed by a rapid decline in concentrations.[7] Despite the relatively short duration of intranasal estradiol, it is has similar effectiveness to other, longer-lasting routes of administration in terms of relief of menopausal symptoms like hot flashes.[7]
Transdermal administration
_per_day_in_postmenopausal_women.png)
_with_and_without_an_ethanol_injection_in_postmenopausal_women.png)
Transdermal estradiol is available in the forms of patches, gels, emulsions, and sprays.[191][192][7][112] In the case of gels, emulsions, and sprays, the route is sometimes referred to as topical rather than as transdermal.[192][190][3] Estradiol patches have an extended duration and are available for twice-weekly (3–4-day) and once-weekly (7-day) application, while gels, emulsions, and sprays are administered daily.[192][43][7][193] There are two types of estradiol patches: reservoir patches, which have been described as first-generation patches, and matrix patches, which are considered to be improved second-generation patches.[7][9][192] Reservoir patches were designed for twice-weekly application, while matrix patches have been produced for both twice-weekly and once-weekly application.[9] Reservoir patches of estradiol (e.g., Estraderm) are mostly no longer used, with most estradiol patches available today being matrix patches (e.g., Alora, Climara, Esclim, Estradot, FemPatch, Menostar, Oesclim, Vivelle, and Vivelle-Dot).[192] When estradiol is administered as a hydroalcoholic gel such as EstroGel, it dries within 2 to 5 minutes following application to the skin.[189]
Estradiol has moderate skin permeability, which is based on the lipophilicity and hydrophilicity of a compound.[7] In general, the more hydroxyl groups that are present in a steroid, the less its skin permeability.[7] For this reason, estrone and progesterone have high skin permeability, while estriol and cortisol have low skin permeability.[7] Regardless of administration form, such as patch or gel, transdermal estradiol is transported into the skin, including through the stratum corneum, epidermis, and dermis, by a passive diffusion process.[7][189] Following this, estradiol is then taken up by local capillary blood vessels and delivered into the circulation.[7] There is a depot effect in the skin with transdermal estradiol, which results in continuous delivery of transdermal estradiol into the circulation.[112][189] This is because the skin functions as a semipermeable membrane and there is a concentration gradient between the application site of transdermal estradiol and capillary blood, with the rate of diffusion of estradiol across the stratum corneum being the specific rate-limiting factor in absorption.[7][189] As a result, peaks and troughs in circulating estradiol levels are limited, and the skin and subcutaneous fat act as a reservoir of estradiol that maintains circulating estradiol levels between doses.[112] For these reasons, transdermal estradiol can provide near-constant circulating levels of estradiol, similarly to oral estradiol.[112][7] A single application of a transdermal estradiol gel (e.g., EstroGel) results in a sustained increase in estradiol levels for at least 24 hours.[112][189] Enzymes that metabolize estradiol are minimally expressed in the skin, and for this reason, the metabolism of estradiol in the skin is low.[7] The apparent elimination half-life of estradiol with transdermal estradiol gel (as EstroGel) is 36 hours.[189]
Transdermal estradiol bypasses the intestines and liver and hence the first-pass metabolism that is associated with oral administration.[7] As a result, it has much greater bioavailability and potency than oral estradiol.[7] In spite of a 20-fold difference in dosage, the circulating levels of estradiol with 2 mg/day oral estradiol and 100 µg/day transdermal estradiol are similar.[139][9] A dosage of 1 mg/day oral estradiol is considered to be roughly equivalent to 50 µg/day transdermal estradiol and a dosage of 2 mg/day oral estradiol is considered to be equivalent to 100 µg/day transdermal estradiol.[148][9][7] In addition, unlike oral estradiol, transdermal estradiol is not associated with supraphysiological concentrations of estrone or estrogen conjugates like estradiol sulfate, and transdermal estradiol does not have disproportionate effects on liver protein synthesis.[7] In accordance, estradiol patches, at typical menopausal replacement dosages, have been found not to increase the risk of blood clots or insulin resistance,[163][9] nor to affect hepatic SHBG, IGF-1, GHBP,[166] IGFBP,[167] and other protein production and by extension circulating hepatic protein levels.[164][165][168]
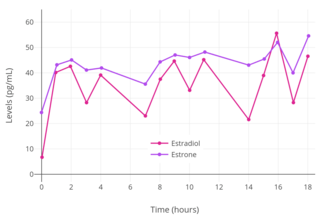
Transdermal administration of estradiol via patch or gel produces an estradiol to estrone ratio of about 1:1.[7][9] Estradiol patches delivering a daily dosage of 0.05 mg (50 µg) achieve mean estradiol and estrone levels of 30 to 65 pg/mL and 40 to 45 pg/mL, respectively, while a daily dosage of 0.1 mg (100 µg) attains respective mean levels of 50 to 90 pg/mL and 30 to 65 pg/mL of estradiol and estrone.[43] Once daily application of 1.25 g topical gel containing 0.75 mg estradiol (brand name EstroGel) for 2 weeks was found to produce mean peak estradiol and estrone levels of 46.4 pg/mL and 64.2 pg/mL, respectively.[189] The time-averaged levels of circulating estradiol and estrone with this formulation over the 24-hour dose interval were 28.3 pg/mL and 48.6 pg/mL, respectively.[189] Levels of estradiol and estrone are stable and change relatively little over the course of the 24 hours following an application, indicating a long duration of action of this route.[189] Steady-state levels of estradiol are achieved after 3 days of application.[189] A higher dosage of estradiol gel containing 1.5 mg estradiol per daily application has been found to produce mean estradiol levels of 40 to 100 pg/mL and estrone levels of 90 pg/mL, while 3 mg per day has been found to result in respective mean estradiol and estrone levels of 60 to 140 pg/mL and 45 to 155 pg/mL.[43] Following removal of an estradiol patch, circulating estradiol levels decrease to baseline within 24 hours.[7]
Typical dosages of estradiol patches are intended to provide the minimum amount of estrogen replacement necessary for the effective alleviation of menopausal symptoms, and for this reason, they achieve relatively low levels of estradiol.[7] A dosage of two to six 100 µg/day transdermal estradiol patches can achieve mean levels of estradiol in the area of 200 to 400 pg/mL and can be used as a form of high-dose estrogen therapy, for instance to suppress testosterone levels in the treatment of prostate cancer in men and in feminizing hormone therapy for transgender women.[11][47][65]
Transdermal estradiol patches are described as delivering a fixed amount of estradiol such as 50 µg/day or 100 µg/day.[7] However, there is large interindividual variability and intraindividual variability`in the pharmacokinetic parameters of transdermal estradiol, and fluctuations in circulating estradiol levels with estradiol patches is almost as great as with oral estradiol.[7][9][112] As such, the actual delivery rate of estradiol and mean levels of estradiol achieved with transdermal estradiol patches may be different from what is described and from the mean levels observed in clinical studies, respectively.[7] A wide range of estradiol levels are measured in women using the same estradiol patch or gel and dosage, with an up to 10-fold difference in levels.[7][112] For example, the interindividual variability with Estraderm reservoir patches ranges from 25 to 225%.[112] In as many as 30% of women treated with a 50 µg/day estradiol patch, estradiol levels are low.[7] There are also significant short-term intraindividual differences in estradiol levels with estradiol patches; estradiol levels can fluctuate considerably from hour to hour.[7][190] In addition, estradiol levels with estradiol patches are higher in the evening than in the morning, which may be due to circadian variations in skin blood flow that may influence absorption.[7] In terms of area-under-the-curve levels of estradiol achieved, the interindividual variability of transdermal estradiol has been found to be 20 to 44% using different transdermal formulations, and the intraindividual variability with transdermal estradiol has been found to be 20%.[9] Factors which may contribute to inter- and intraindividual variability with transdermal estradiol include skin location and thickness; hair follicle density; solvent (alcohol) evaporation; skin dehydration, ambient temperature, and humidity; and reservoir size.[112]
Estradiol patches are associated with local skin reactions and such as irritation in 14.2% of individuals (with reservoir patches), mild-to-moderate erythema (redness) in 50 to 60% of individuals, and allergic reactions due to cutaneous sensitization.[7][9] Up to 5% of people using reservoir patches may discontinue therapy due to skin reactions.[9] Visible adhesive residues are also often left by estradiol patches following their removal.[7]
| Brand name |
Forms (µg/day) | Duration | Type | Size (cm2) | Estradiol (mg) | Levels (pg/mL) |
Launch (year) |
Hits | Refs |
|---|---|---|---|---|---|---|---|---|---|
| Alora | 25, 50, 75, 100 | 3–4 days | Matrix | 9, 18, 27, 36 | 0.77, 1.5, 2.3, 3.1 | 43–144 | 1996 | 42,300 | [194][195] |
| Climara | 25, 37.5, 50, 60, 75, 100 | 7 days | Matrix | 6.5, 9.375, 12.5, 15, 18.75, 25 | 2, 2.85, 3.8, 4.55, 5.7, 7.6 | 17–174 | 1994 | 110,000 | [196][186] |
| Climara Proa | E2 (45) + LNG (15) | 7 days | Matrix | 22 | 4.4 | 27–54 | 2003 | 23,400 | [197][198] |
| CombiPatcha | E2 (50) + NETA (14, 25) | 3–4 days | Matrix | 9, 16 | 0.62, 0.51 | 27–71 | 1998 | 33,500 | [199][200] |
| Esclimc | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 11, 16.5, 22, 33, 44 | 5, 7.5, 10, 15, 20 | 16–124 | 1998 | 31,800 | [201][202] |
| Estradermc | 50, 100 | 3–4 days | Reservoir | 10, 20 | E2 (4, 8 mg) + EtOH (0.3, 0.6 mL) | 32–73 | 1986 | 60,200 | [203][204] |
| Estradiolb | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 2.5, 3.75, 5, 7.5, 10 | 0.41, 0.62, 0.82, 1.23, 1.64 | 30–145 | 1996 | – | [205][185] |
| Estradiolb | 25, 37.5, 50, 75, 100 | 7 days | Matrix | 7.75, 11.625, 15.5, 18.6, 23.25, 31 | 0.97, 1.46, 1.94, 2.33, 2.91, 3.88 | 17–174 | 2000 | – | [206][188] |
| FemPatchc | 25 | 7 days | Matrix | 30 | ND | ND | ND | 18,200 | [207] |
| Menostar | 14 | 7 days | Matrix | 3.25 | 1 | 13–21 | 2004 | 21,300 | [208][187] |
| Minivelle | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 1.65, 2.48, 3.3, 4.95, 6.6 | 0.41, 0.62, 0.83, 1.24, 1.65 | 30–117 | 2012 | 15,100 | [209][210] |
| Vivelled | 3–4 days | Matrix | 30–145 | 2000 | 91,900 | [211][183] | |||
| Vivelle-Dot | 25, 37.5, 50, 75, 100 | 3–4 days | Matrix | 2.5, 3.75, 5, 7.5, 10 | 0.39, 0.585, 0.78, 1.17, 1.56 | 30–145 | 1996 | 68,900 | [212][184] |
| a = Combined with a progestin. b = Generic (of Vivelle-Dot, Climara; by Mylan). c = Discontinued. d = Partially discontinued (strikethroughed are the specific discontinued forms). | |||||||||
Vaginal administration
Vaginal estradiol is available in the forms of tablets, creams, inserts or suppositories, and rings.[192][7][191] Vaginal estradiol tablets, creams, and inserts are usually administered once daily to twice weekly, whereas vaginal estradiol rings have a sustained action and are replaced once every 90 days.[192][7] Vaginal estradiol can be used both as a systemic form of estradiol therapy, and at very low doses to selectively achieve a local vaginal effect without systemic effects, for instance in the treatment of menopausal symptoms such as vaginal atrophy and dryness.[7][213]
Vaginal estradiol is rapidly and almost completely absorbed.[171] The absorption of vaginal estradiol is slightly greater in women with vaginal atrophy.[171] Similarly to transdermal estradiol, vaginal estradiol has greatly increased potency compared to oral estradiol, with about 10- to 20-fold the comparative potency of oral estradiol.[7] The greater potency of vaginal estradiol relative to oral estradiol is due to the lack of the first pass associated with oral estradiol and due to local metabolism of estradiol in the vagina being low.[7] Because of the high estradiol levels achieved, LH and FSH are more strongly suppressed with vaginal estradiol than with other routes.[171]
A daily dosage of 0.5 mg vaginal micronized estradiol has been found to result in estradiol and estrone levels of 250 pg/mL and 130 pg/mL, respectively.[43] Vaginal estradiol has a much higher estradiol-to-estrone ratio in comparison to oral estradiol.[7] The average ratio of estradiol to estrone with vaginal estradiol is 5:1, compared to 1:5 in the case of oral estradiol, a 10-fold difference.[7]
As vaginal estradiol is not subject to a first pass and bypasses the intestines and liver, it does not affect liver protein synthesis at menopausal replacement dosages, similarly to transdermal estradiol.[214]
Rectal administration
Estradiol has been studied for use by rectal administration.[215][216][217][218][219] Rectal administration of estradiol is described as qualitatively and quantitatively similar to vaginal administration of estradiol.[216][171] Lauritzen (1986) reported that 3 hours after a rectal dose of 1 mg micronized estradiol, estradiol levels increased by 620 pg/mL and estrone levels increased by 120 pg/mL.[216][171] Subsequently, Lauritzen (1990) reported that 0.5 mg/day and 1 mg/day rectal estradiol resulted in respective estradiol levels of 363 pg/mL and 515 pg/mL 6 hours after the last dose.[215] These estradiol levels are fairly similar to those achieved by vaginal estradiol.[216][215][171] The estradiol-to-estrone ratio of rectal estradiol is about 5:1, which is the same as that of vaginal estradiol.[216][171] Absorption of rectal estradiol occurs rapidly within 30 to 60 minutes, maximal estradiol levels occur at 3 hours post-dose, and circulating estradiol levels are reportedly maintained for 4 to 10 hours.[216][171] The duration of rectal estradiol is said to necessitate administration 1 to 2 times per day.[216] Irritation of the intestines does not usually occur.[216] Rectal administration of estradiol is not accepted by all individuals.[216]
Rectal estradiol has been studied as a form of high-dose estrogen therapy for the treatment of prostate cancer in men.[218]
Rectal administration of estriol, which has similar properties to estradiol, has also been studied.[220] Administration of a rectal suppository containing 100 mg estriol resulted in estriol levels in pregnant women at term increasing by about 53%.[220] Estriol levels at term are normally between 5,000 and 20,000 pg/mL.[221][222][223]
Intramuscular injection
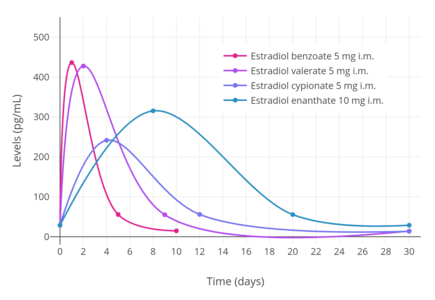
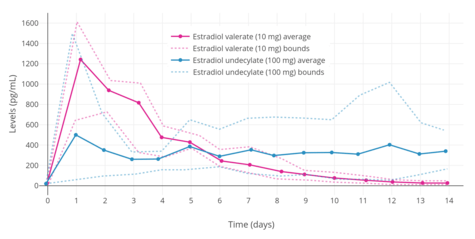
Estradiol, in an ester prodrug form such as estradiol valerate or estradiol cypionate, can be administered by intramuscular injection, via which a long-lasting depot effect occurs.[125][6] In contrast to the oral route, the bioavailability of estradiol and its esters like estradiol valerate is complete (i.e., 100%) with intramuscular injection.[2] The levels of estradiol that are achieved with typical clinical dosages of injections are very high compared to other routes.[7][111][225][125][10]
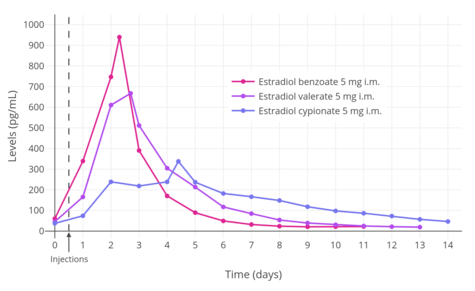
A single 4 mg intramuscular injection of estradiol cypionate or estradiol valerate has been found to result in maximal plasma levels of estradiol of about 250 pg/mL and 390 pg/mL, respectively, with levels declining to 100 pg/mL (the baseline for estradiol cypionate) by 12 to 14 days.[111][225] A single 2.5 mg intramuscular injection of estradiol benzoate in patients being administered a GnRH analogue (and hence having minimal baseline levels of estrogen) was found to result in serum estradiol levels of >400 pg/mL at 24 hours post-administration.[125] The differences in the serum levels of estradiol achieved with these different estradiol esters may be explained by their different rates of absorption, as their durations and levels attained appear to be inversely proportional.[125] For instance, estradiol benzoate, which has the shortest duration (4 to 5 days with a single intramuscular injection of 5 mg), produces the highest levels of estradiol, while estradiol cypionate, which has the longest duration (~11 days with 5 mg), produces the lowest levels of estradiol.[125] Estradiol valerate was found to have a duration of 7 to 8 days after a single intramuscular injection of 5 mg.[125]
A study of combined high-dosage intramuscular estradiol valerate and hydroxyprogesterone caproate in peri- and postmenopausal and hypogonadal women (a pseudopregnancy regimen), with specific dosages of 40 mg weekly and 250 mg weekly, respectively, was found to result in serum estradiol levels of 3,028 to 3,226 pg/mL after three months and 2,491 to 2,552 pg/mL after six months of treatment from a baseline of 27.8 to 34.8 pg/mL.[226]
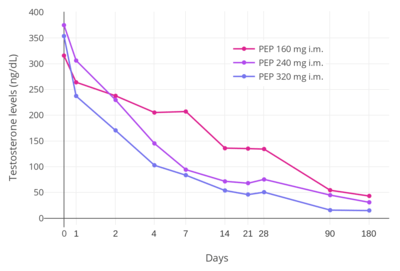
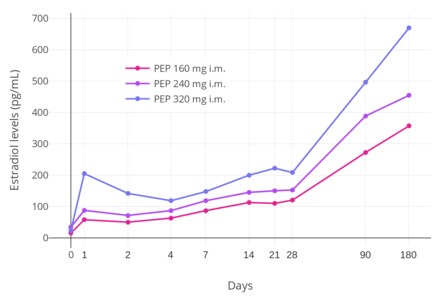
Polyestradiol phosphate is an ester prodrug of estradiol in the form of a polymer which is used via intramuscular injection primarily to treat prostate cancer, but also to treat breast cancer and menopausal symptoms.[227][228] It has an extremely long duration of action, with a terminal half-life of about 70 days (10 weeks) following a single intramuscular administration of the medication.[46] In addition, unlike most other estradiol esters, the estradiol levels achieved with polyestradiol phosphate are highly constant and uniform.[46] Levels of estradiol in men with intramuscular injections of polyestradiol phosphate once every 4 weeks were about 350 pg/mL with 160 mg, 450 pg/mL with 240 mg, and almost 700 pg/mL with 320 mg, all measured after 6 months of treatment.[10] Polyestradiol phosphate has been discontinued in many countries, and remains available today in only a few countries, mostly the Nordic countries of Europe.[228][229]
Estradiol is available in the form of an aqueous suspension of 1.0 mg estradiol encapsulated in microspheres for use by intramuscular injection once a month under the brand name Juvenum E in Mexico.[230][231] It achieves circulating estradiol levels of 163 pg/mL to 219 pg/mL in the first 3 to 12 hours following injection, which decrease to 42.4 to 65.7 pg/mL during the first 4 days post-injection and to 20 to 35 pg/mL after 8 days, with levels remaining in this range thereafter over 30 days.[230] These estradiol levels are similar to the normal levels that occur during the early follicular phase of the menstrual cycle in premenopausal women (24 to 75 pg/mL).[230] The elimination of the formulation follows three phases: a rapid phase in the first 2 days, a second phase during days 2 to 12 days with a biological half-life of 7.2 to 9.9 days, and a third phase in which estradiol levels remain elevated above baseline for up to 30 days.[230]
Subcutaneous injection
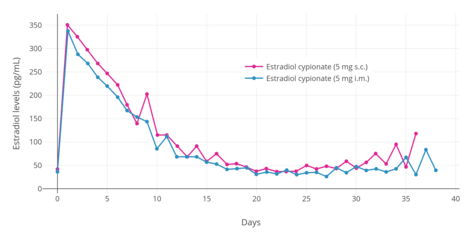
Estradiol esters like estradiol valerate and estradiol cypionate can be given by subcutaneous injection instead of intramuscular injection.[232] Subcutaneous and intramuscular injection of estradiol cypionate in an aqueous suspension has been found to result in levels of estradiol and other pharmacokinetic parameters (e.g., duration) that were virtually identical.[6] However, subcutaneous injections may be easier and less painful to perform compared to intramuscular injections, and hence may result in improved compliance and satisfaction.[6] Studies have shown that subcutaneous injection of closely related steroid esters in oil like the androgen esters testosterone cypionate, testosterone enantate, and nandrolone decanoate is effective and has similar pharmacokinetics to intramuscular injection as well.[233][65][234][235][236][237][238] In addition, studies have found that many intramuscular injections are really subcutaneous injections, as people often do not actually penetrate deep enough to inject into muscle.[239][240] This is particularly prevalent with injections into the buttocks and in overweight and obese individuals, due to the thicker fat layer over muscle.[239][240]
Subcutaneous implant
Estradiol can be administered in the form of a very long-lasting subcutaneous pellet implant.[7][241] These implants are replaced once every 6 to 12 months, and can achieve high and very constant circulating levels of estradiol.[7][242][243] They are surgically inserted by a trained physician in a medical office or clinic and can be placed into locations including the lower abdomen, lower back, buttocks, or hips.[7][242][241] Subcutaneous implants containing 25, 50, and 100 mg estradiol for replacement usually once every 6 months are available as approved pharmaceutical medications.[243] Pharmaceutical estradiol implants have been said to be used almost exclusively in the United Kingdom.[244] An estradiol implant has not been approved by the FDA as a pharmaceutical medication in the United States, but hormone implants, including estradiol implants, are available as custom compounded products in this country.[245][246][247]
Estradiol implants are advantageous in that some women seem to need higher levels of estradiol for adequate relief of menopausal symptoms, and subcutaneous implants are easily able to achieve such levels.[243][7] Conversely, this is not necessarily the case with oral or transdermal estradiol.[243][7] Another major advantage of estradiol implants is convenience and guaranteed compliance.[243] They also do not have the issues pertaining to first-pass metabolism and liver protein synthesis of oral estradiol.[243][7] A major disadvantage of estradiol implants is that they cannot be easily removed should this be necessary.[243] There are also concerns about accumulation of estradiol levels with long-term repeated pellet implantation.[243][7] Estradiol levels may remain above baseline for a year or in some cases 3 to 4 years following the last implant insertion.[243] During this time, progestogen therapy should be continued to avoid the risk of endometrial changes.[243][242] Regular monitoring of estradiol levels and adjustment of dosing is recommended during therapy with estradiol implants.[243]
Tachyphylaxis of relief of vasomotor symptoms, or hot flashes returning even with normal supraphysiological estradiol levels, may occur in a small subset of cases with subcutaneous estradiol implants.[243][7][244][248][242] The reason for this is unknown, but may be a paradoxical effect of the high levels of estradiol achieved and/or a product of receptor desensitization caused by the long-term gradually decreasing levels of estradiol.[243][7] Such symptoms reportedly do not start to occur until estradiol levels begin to decrease, although there are also reports of them occurring 3 to 16 weeks (1 to 4 months) after implant insertion when levels should still be constant.[243][7] Hot flashes have notably been reported in pregnant women, who have very high levels of estradiol.[249] When recurrence of hot flashes occurs with estradiol implants, treated women often complain that their implant has "run out".[243] Such symptoms can be temporarily offset with the use of oral or transdermal estradiol.[243]
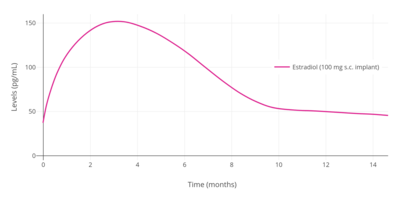
Following insertion of an estradiol implant, levels of estradiol rapidly increase, remain constant for about 4 months, and then gradually decrease.[243] A 25 mg subcutaneous estradiol pellet has been found to result in average estradiol levels of 90 pg/mL for 6 months, while two 25 mg pellets (50 mg total) resulted in estradiol levels of 180 pg/mL after 24 hours and levels of 100 to 120 pg/mL for 6 months.[7] Higher-dose implants resulted in estradiol levels for 50 mg of 100 pg/mL, for 75 mg of 140 pg/mL, and for 100 mg of 150 pg/mL.[7] Estradiol levels are generally 50% higher than those of estrone, for an estradiol-to-estrone ratio of 1.5:1.[7] Extremely high levels of estradiol of between 400 to 1,000 pg/mL have been observed in a small subset of women treated with estradiol implants and notably in those experiencing symptoms of tachyphylaxis.[243][7]
General (ADME)
Absorption
Estradiol is well-absorbed regardless of route of administration.[7] However, the bioavailability of estradiol differs substantially with different routes of administration.[7][2] Oral estradiol has an average bioavailability of around 5%, requiring relatively high dosages of estradiol for effects.[7] Conversely, transdermal estradiol has much higher potency, with a 20-fold lower dosage of transdermal estradiol being equivalent in potency to oral estradiol.[7][139][9] Estradiol administered in the form of an ester by intramuscular or subcutaneous injection has complete bioavailability.[2][232][6]
Distribution
Estradiol is rapidly distributed throughout the body, with a distribution phase of about 6 minutes following intravenous injection.[9] Due to binding to the ERs, estradiol is preferentially concentrated in tissues with the highest ER content.[9] In animals, these tissues have included the pituitary gland, hypothalamus, other brain regions, uterus, liver, vagina, and adrenals, among other tissues.[9] In contrast to estradiol, likely due to its low affinities for the ERs, estrone is not accumulated in target tissues.[7] Estradiol has been found to cross the blood–brain barrier in rhesus monkeys.[9] The volume of distribution of estradiol has been found to be 0.85 to 1.17 L/kg.[9]
In terms of plasma protein binding, estradiol is bound loosely to albumin and tightly to SHBG, with approximately 97 to 98% of estradiol bound to plasma proteins.[136] In the circulation, approximately 38% of estradiol is bound to SHBG and 60% is bound to albumin, with 2 to 3% free or unbound.[134] However, with oral estradiol, there is an increase in hepatic SHBG production and hence SHBG levels (e.g., +50%), and this results in a relatively reduced fraction of free estradiol.[1][112] As only free estradiol that is not bound to plasma proteins or SHBG is biologically active, this may reduce the potency of oral estradiol by some degree.[9][112]
Metabolism
There are several major pathways of estradiol metabolism, which occur both in the liver and in other tissues:[9][7][1]
- Dehydrogenation by 17β-hydroxysteroid dehydrogenase (17β-HSD) into estrone
- Conjugation by estrogen sulfotransferases and UDP-glucuronyltransferases into C3 and/or C17β estrogen conjugates like estrone sulfate and estradiol glucuronide
- Hydroxylation by cytochrome P450 enzymes such as CYP1A1 and CYP3A4 into catechol estrogens like 2-hydroxyestrone and 2-hydroxyestradiol as well as 16-hydroxylated estrogens like 16α-hydroxyestrone and estriol (16α-hydroxyestradiol)
Both dehydrogenation of estradiol by 17β-HSD into estrone and conjugation into estrogen conjugates are reversible transformations.[9][7] However, in regards to sulfation and desulfation, transformation of estrone into estrone sulfate is predominant relative to the reverse reaction.[9][157]
Estradiol can also be reversibly converted into long-lived lipoidal estradiol forms like estradiol palmitate and estradiol stearate as a minor route of metabolism.[8]
The terminal half-life of estradiol administered via intravenous injection is 2 hours in men and 50 minutes in women.[2] Other routes of administration of estradiol like oral administration or intramuscular injection have far longer terminal half-lives and durations of action due to (1) the formation of a large circulating reservoir of metabolism-resistant estrogen conjugates that can be reconverted back into estradiol and/or (2) the formation of slowly-releasing depots.[9][7]
The metabolic clearance rates of estradiol, estrone, and estrone sulfate are 580 L/day/m2, 1,050 L/day/m2, and 80 L/day/m2, respectively.[7]
Elimination
A single dose of oral estradiol valerate is eliminated 54% in urine and 6% in feces.[1] A substantial amount of estradiol is also excreted in bile.[1] The urinary metabolites of estradiol are predominantly present in the form of estrogen conjugates, including glucuronides and, to a lesser extent, sulfates.[1] The main metabolites of estradiol in urine are estrone glucuronide (13–30%), 2-hydroxyestrone (2.6–10.1%), unchanged estradiol (5.2–7.5%), estriol (2.0–5.9%), and 16α-hydroxyestrone (1.0–2.9%).[1]
See also
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- 1 2 3 4 5 6 7 8 9 Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. PMID 7169965.
- 1 2 3 Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 0-323-03309-1.
- ↑ Price, T; Blauer, K; Hansen, M; Stanczyk, F; Lobo, R; Bates, G (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17-estradiol". Obstetrics & Gynecology. 89 (3): 340–345. doi:10.1016/S0029-7844(96)00513-3. ISSN 0029-7844.
- ↑ Naunton, Mark; Al Hadithy, Asmar F. Y.; Brouwers, Jacobus R. B. J.; Archer, David F. (2006). "Estradiol gel". Menopause. 13 (3): 517–527. doi:10.1097/01.gme.0000191881.52175.8c. ISSN 1072-3714.
- 1 2 3 4 5 6 Sierra-Ramírez JA, Lara-Ricalde R, Lujan M, Velázquez-Ramírez N, Godínez-Victoria M, Hernádez-Munguía IA, et al. (2011). "Comparative pharmacokinetics and pharmacodynamics after subcutaneous and intramuscular administration of medroxyprogesterone acetate (25 mg) and estradiol cypionate (5 mg)". Contraception. 84 (6): 565–70. doi:10.1016/j.contraception.2011.03.014. PMID 22078184.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 55 56 57 58 59 60 61 62 63 64 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- 1 2 3 4 5 6 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. pp. 121, 226, 235–237. ISBN 978-3-642-58616-3.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 163–178, 235–237, 252–253, 261–276, 538–543. ISBN 978-3-642-60107-1.
- 1 2 3 4 5 6 7 8 9 10 11 Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384.
- 1 2 3 4 5 6 7 Ockrim JL, Lalani EN, Laniado ME, Carter SS, Abel PD (2003). "Transdermal estradiol therapy for advanced prostate cancer--forward to the past?". J. Urol. 169 (5): 1735–7. doi:10.1097/01.ju.0000061024.75334.40. PMID 12686820.
- 1 2 3 Escande A, Pillon A, Servant N, Cravedi JP, Larrea F, Muhn P, Nicolas JC, Cavaillès V, Balaguer P (2006). "Evaluation of ligand selectivity using reporter cell lines stably expressing estrogen receptor alpha or beta". Biochem. Pharmacol. 71 (10): 1459–69. doi:10.1016/j.bcp.2006.02.002. PMID 16554039.
- ↑ Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson J, Nilsson S (July 1998). "Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists". Mol. Pharmacol. 54 (1): 105–12. doi:10.1124/mol.54.1.105. PMID 9658195.
- ↑ Soltysik K, Czekaj P (April 2013). "Membrane estrogen receptors – is it an alternative way of estrogen action?". J. Physiol. Pharmacol. 64 (2): 129–42. PMID 23756388.
- ↑ Prossnitz ER, Barton M (May 2014). "Estrogen biology: New insights into GPER function and clinical opportunities". Mol. Cell. Endocrinol. 389 (1–2): 71–83. doi:10.1016/j.mce.2014.02.002. PMC 4040308. PMID 24530924.
- 1 2 Ojasoo T, Raynaud JP (November 1978). "Unique steroid congeners for receptor studies". Cancer Res. 38 (11 Pt 2): 4186–98. PMID 359134.
- 1 2 Ojasoo T, Delettré J, Mornon JP, Turpin-VanDycke C, Raynaud JP (1987). "Towards the mapping of the progesterone and androgen receptors". J. Steroid Biochem. 27 (1–3): 255–69. doi:10.1016/0022-4731(87)90317-7. PMID 3695484.
- 1 2 Raynaud JP, Bouton MM, Moguilewsky M, Ojasoo T, Philibert D, Beck G, Labrie F, Mornon JP (January 1980). "Steroid hormone receptors and pharmacology". J. Steroid Biochem. 12: 143–57. doi:10.1016/0022-4731(80)90264-2. PMID 7421203.
- 1 2 3 Raynaud, J.P.; Ojasoo, T.; Bouton, M.M.; Philibert, D. (1979). "Receptor Binding as a Tool in the Development of New Bioactive Steroids": 169–214. doi:10.1016/B978-0-12-060308-4.50010-X.
- 1 2 3 Blankvoort BM, de Groene EM, van Meeteren-Kreikamp AP, Witkamp RF, Rodenburg RJ, Aarts JM (November 2001). "Development of an androgen reporter gene assay (AR-LUX) utilizing a human cell line with an endogenously regulated androgen receptor". Anal. Biochem. 298 (1): 93–102. doi:10.1006/abio.2001.5352. PMID 11673900.
- ↑ Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 495–. ISBN 978-1-107-01290-5.
- 1 2 Goldstein I, Meston CM, Davis S, Traish A (17 November 2005). Women's Sexual Function and Dysfunction: Study, Diagnosis and Treatment. CRC Press. pp. 205–, 540. ISBN 978-1-84214-263-9.
- ↑ Robert Marcus; David W. Dempster; Jane A. Cauley; David Feldman (13 June 2013). Osteoporosis. Academic Press. pp. 1117–. ISBN 978-0-12-398252-0.
Altogether, men make 20-fold more androgens than do women; the proportion of androgen converted to E2 is 200-fold more in women; and E2 is 1000-fold more potent than androgens (on a molar basis) on target tissues [28]. Thus, circulating estrogen levels are measured in picograms, and testosterone levels are measured in nanograms.
- ↑ Thomas, John A.; Keenan, Edward J. (1986). "Estrogens and Antiestrogenic Drugs": 135–165. doi:10.1007/978-1-4684-5036-1_7.
Cytoplasmic estrogen receptors characteristically exhibit high affinity for estradiol-17J3, with an equilibrium dissociation constant of 0.1 nM. The number of these sites in target tissues is generally low, approximating 10,000-20,000 sites per cell.
- 1 2 Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". J. Urol. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
In cell culture37 and gonadectomized rodents48 the addition of E can induce the autoregulation of ERs. This finding suggests that the ER expression depends on the level of serum E and to maintain an effective cellular response to E2 regulation of the ER is crucial. Prolonged E2 administration at a constant dose may not be maximally effective for patients with PCa. As a result of continuous exposure, ERs may be down-regulated, attenuating their effectiveness. Thus, cyclical rather than continuous administration of E may be preferable.
- 1 2 3 Nawaz Z, Lonard DM, Dennis AP, Smith CL, O'Malley BW (March 1999). "Proteasome-dependent degradation of the human estrogen receptor". Proc. Natl. Acad. Sci. U.S.A. 96 (5): 1858–62. doi:10.1073/pnas.96.5.1858. PMC 26701. PMID 10051559.
- ↑ Miller, Colette (October 2015). "A brief on the structure and function of estrogen receptor alpha (BCMB8010 Enzyme Project)". doi:10.13140/RG.2.1.4082.5044.
ERα is relatively stable in the cell with a half-life of up to 5 days, however once bound to ligand this time shortens to 3-4 hours.
- 1 2 Kloosterboer, Helenius; Schoonen, Willem; Verheul, Herman (2008). "Proliferation of Breast Cells by Steroid Hormones and Their Metabolites": 343–366. doi:10.3109/9781420058734-19.
Steroid deprivation, for instance, can have a major impact on the growth stimulation by E2. Estrogen sensitivity can be increased easily by four log-units or more (Masamura et al., 1995; Chan et al., 2002) (Fig. 1). This effect may be explained, at least partly, by a 100-fold higher level of ER(s) (Zajchowski et al., 1993), but coactivator sensitivity as well as the degree of phosphorylation of transactivation factors (TAF-1 and/or TAF-2) may also be crucial.
- ↑ Mauvais-Jarvis P, Kuttenn F, Gompel A (1986). "Antiestrogen action of progesterone in breast tissue". Breast Cancer Res. Treat. 8 (3): 179–88. doi:10.1007/BF01807330. PMID 3297211.
- ↑ Zhou J, Ng S, Adesanya-Famuiya O, Anderson K, Bondy CA (September 2000). "Testosterone inhibits estrogen-induced mammary epithelial proliferation and suppresses estrogen receptor expression". FASEB J. 14 (12): 1725–30. doi:10.1096/fj.99-0863com. PMID 10973921.
- ↑ Dunn JF, Nisula BC, Rodbard D (July 1981). "Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 58–68. doi:10.1210/jcem-53-1-58. PMID 7195404.
- ↑ Pugeat MM, Dunn JF, Nisula BC (July 1981). "Transport of steroid hormones: interaction of 70 drugs with testosterone-binding globulin and corticosteroid-binding globulin in human plasma". J. Clin. Endocrinol. Metab. 53 (1): 69–75. doi:10.1210/jcem-53-1-69. PMID 7195405.
- 1 2 Fritz F. Parl (2000). Estrogens, Estrogen Receptor and Breast Cancer. IOS Press. pp. 4, 111. ISBN 978-0-9673355-4-4.
- ↑ Jennifer E. Dietrich (18 June 2014). Female Puberty: A Comprehensive Guide for Clinicians. Springer. pp. 53–. ISBN 978-1-4939-0912-4.
- ↑ Randy Thornhill; Steven W. Gangestad (25 September 2008). The Evolutionary Biology of Human Female Sexuality. Oxford University Press. pp. 145–. ISBN 978-0-19-988770-5.
- ↑ Raine-Fenning NJ, Brincat MP, Muscat-Baron Y (2003). "Skin aging and menopause : implications for treatment". Am J Clin Dermatol. 4 (6): 371–8. PMID 12762829.
- ↑ Chris Hayward (31 July 2003). Gender Differences at Puberty. Cambridge University Press. pp. 22–. ISBN 978-0-521-00165-6.
- ↑ Shlomo Melmed; Kenneth S. Polonsky; P. Reed Larsen; Henry M. Kronenberg (11 November 2015). Williams Textbook of Endocrinology. Elsevier Health Sciences. pp. 1105–. ISBN 978-0-323-34157-8.
- ↑ Richard E. Jones; Kristin H. Lopez (28 September 2013). Human Reproductive Biology. Academic Press. pp. 19–. ISBN 978-0-12-382185-0.
- 1 2 3 4 5 Waun Ki Hong; James F. Holland (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 753–. ISBN 978-1-60795-014-1.
- ↑ Ethel Sloane (2002). Biology of Women. Cengage Learning. pp. 496–. ISBN 0-7668-1142-5.
- ↑ Tekoa L. King; Mary C. Brucker (25 October 2010). Pharmacology for Women's Health. Jones & Bartlett Learning. pp. 1022–. ISBN 978-0-7637-5329-0.
- 1 2 3 4 5 6 7 8 9 10 11 12 Rogerio A. Lobo (5 June 2007). Treatment of the Postmenopausal Woman: Basic and Clinical Aspects. Academic Press. pp. 177, 217–226, 770–771. ISBN 978-0-08-055309-2.
- ↑ David Warshawsky; Joseph R. Landolph Jr. (31 October 2005). Molecular Carcinogenesis and the Molecular Biology of Human Cancer. CRC Press. pp. 457–. ISBN 978-0-203-50343-0.
- ↑ Acevedo-Rodriguez A, Mani SK, Handa RJ (2015). "Oxytocin and Estrogen Receptor β in the Brain: An Overview". Frontiers in Endocrinology. 6: 160. doi:10.3389/fendo.2015.00160. PMC 4606117. PMID 26528239.
- 1 2 3 4 5 6 7 Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057.
- 1 2 Langley RE, Godsland IF, Kynaston H, Clarke NW, Rosen SD, Morgan RC, Pollock P, Kockelbergh R, Lalani EN, Dearnaley D, Parmar M, Abel PD (August 2008). "Early hormonal data from a multicentre phase II trial using transdermal oestrogen patches as first-line hormonal therapy in patients with locally advanced or metastatic prostate cancer". BJU Int. 102 (4): 442–5. doi:10.1111/j.1464-410X.2008.07583.x. PMC 2564109. PMID 18422771.
Available information suggests that for preparations delivering 100 µg/day of oestradiol transdermally (including the Progynova [TS forte] patches used in the original pilot study [5]) [...]
- ↑ Ockrim J, Lalani E, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433.
- 1 2 3 4 Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- 1 2 3 4 5 Leinung MC, Feustel PJ, Joseph J (2018). "Hormonal Treatment of Transgender Women with Oral Estradiol". Transgend Health. 3 (1): 74–81. doi:10.1089/trgh.2017.0035. PMC 5944393. PMID 29756046.
- 1 2 Scherr DS, Pitts WR (2003). "The nonsteroidal effects of diethylstilbestrol: the rationale for androgen deprivation therapy without estrogen deprivation in the treatment of prostate cancer". J. Urol. 170 (5): 1703–8. doi:10.1097/01.ju.0000077558.48257.3d. PMID 14532759.
- 1 2 Coss, Christopher C.; Jones, Amanda; Parke, Deanna N.; Narayanan, Ramesh; Barrett, Christina M.; Kearbey, Jeffrey D.; Veverka, Karen A.; Miller, Duane D.; Morton, Ronald A.; Steiner, Mitchell S.; Dalton, James T. (2012). "Preclinical Characterization of a Novel Diphenyl Benzamide Selective ERα Agonist for Hormone Therapy in Prostate Cancer". Endocrinology. 153 (3): 1070–1081. doi:10.1210/en.2011-1608. ISSN 0013-7227. PMID 22294742.
- ↑ Novara G, Galfano A, Secco S, Ficarra V, Artibani W (2009). "Impact of surgical and medical castration on serum testosterone level in prostate cancer patients". Urol. Int. 82 (3): 249–55. doi:10.1159/000209352. PMID 19440008.
- 1 2 3 4 5 6 7 von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function--metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738.
- ↑ Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (25 August 2011). Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. pp. 2938–. ISBN 978-1-4160-6911-9.
- ↑ Knuth UA, Hano R, Nieschlag E (1984). "Effect of flutamide or cyproterone acetate on pituitary and testicular hormones in normal men". J. Clin. Endocrinol. Metab. 59 (5): 963–9. doi:10.1210/jcem-59-5-963. PMID 6237116.
- ↑ Sander S, Nissen-Meyer R, Aakvaag A (1978). "On gestagen treatment of advanced prostatic carcinoma". Scand. J. Urol. Nephrol. 12 (2): 119–21. PMID 694436.
- ↑ Kjeld JM, Puah CM, Kaufman B, Loizou S, Vlotides J, Gwee HM, Kahn F, Sood R, Joplin GF (1979). "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clin. Endocrinol. (Oxf). 11 (5): 497–504. PMID 519881.
- ↑ Gokhan Ozyigit; Ugur Selek (1 August 2017). Principles and Practice of Urooncology: Radiotherapy, Surgery and Systemic Therapy. Springer. pp. 334–. ISBN 978-3-319-56114-1.
The castrate level was defined as testosterone being less than 50 ng/dL (1.7 nmol/L), many years ago. However contemporary laboratory testing methods showed that the mean value after surgical castration is 15 ng/dL [1]. Thus, recently the level is defined as being less than 20 ng/dL (1 nmol/L).
- ↑ Lycette JL, Bland LB, Garzotto M, Beer TM (2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- ↑ Altwein, J. (1983). "Controversial Aspects of Hormone Manipulation in Prostatic Carcinoma": 305–316. doi:10.1007/978-1-4684-4349-3_38.
- ↑ Ockrim JL; Lalani el-N; Kakkar AK; Abel PD (August 2005). "Transdermal estradiol therapy for prostate cancer reduces thrombophilic activation and protects against thromboembolism". J. Urol. 174 (2): 527–33, discussion 532–3. doi:10.1097/01.ju.0000165567.99142.1f. PMID 16006886.
- ↑ Moore E, Wisniewski A, Dobs A (2003). "Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects". J. Clin. Endocrinol. Metab. 88 (8): 3467–73. doi:10.1210/jc.2002-021967. PMID 12915619.
- ↑ Tangpricha V, den Heijer M (2017). "Oestrogen and anti-androgen therapy for transgender women". Lancet Diabetes Endocrinol. 5 (4): 291–300. doi:10.1016/S2213-8587(16)30319-9. PMID 27916515.
- 1 2 3 Deutsch MB, Bhakri V, Kubicek K (2015). "Effects of cross-sex hormone treatment on transgender women and men". Obstet Gynecol. 125 (3): 605–10. doi:10.1097/AOG.0000000000000692. PMC 4442681. PMID 25730222.
- 1 2 Taxel P, Kennedy D, Fall P, Willard A, Shoukri K, Clive J, Raisz LG (2000). "The effect of short-term treatment with micronized estradiol on bone turnover and gonadotrophins in older men". Endocr. Res. 26 (3): 381–98. PMID 11019903.
- 1 2 Dukes, M.N.G. (2002). "Sex hormones and related compounds, including hormonal contraceptives". 25: 478–502. doi:10.1016/S0378-6080(02)80047-2. ISSN 0378-6080.
- 1 2 3 McDowell, Julie. Encyclopedia of Human Body Systems. ABC-CLIO. pp. 201–. ISBN 978-0-313-39175-0.
- 1 2 Herbison AE (June 1998). "Multimodal influence of estrogen upon gonadotropin-releasing hormone neurons". Endocr. Rev. 19 (3): 302–30. doi:10.1210/edrv.19.3.0332. PMID 9626556.
For the greater part of the ovarian cycle, estrogen helps restrain LH secretion through what has been termed its “negative feedback” action. This has been shown to occur, in part, through an inhibition of GnRH secretion in several species (7, 11–13), but also involves potent actions of estrogen on the pituitary gonadotrophs (3, 4, 14). Estrogen also exhibits a “positive feedback” influence upon the GnRH neurons and pituitary gonadotrophs to generate the preovulatory LH surge.
- ↑ Jerome Frank Strauss; Robert L. Barbieri (1 January 2009). Yen and Jaffe's Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Elsevier Health Sciences. pp. 807–. ISBN 1-4160-4907-X.
- ↑ Anand Kumar; Mona Sharma (24 July 2017). Basics of Human Andrology: A Textbook. Springer. pp. 395–. ISBN 978-981-10-3695-8.
- ↑ Miranda A. Farage; Howard I. Maibach (27 March 2017). The Vulva: Physiology and Clinical Management, Second Edition. CRC Press. pp. 139–. ISBN 978-1-4987-5245-9.
- ↑ Rogerio A. Lobo; David M Gershenson; Gretchen M Lentz; Fidel A Valea (22 June 2016). Comprehensive Gynecology E-Book. Elsevier Health Sciences. pp. 97–. ISBN 978-0-323-43003-6.
- ↑ Linda Garnets; Douglas Kimmel (6 May 2003). Psychological Perspectives on Lesbian, Gay, and Bisexual Experiences. Columbia University Press. pp. 62–. ISBN 978-0-231-50494-2.
- ↑ Leon Speroff; Marc A. Fritz (2005). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 211–. ISBN 978-0-7817-4795-0.
- 1 2 Stefan Offermanns; W. Rosenthal (14 August 2008). Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. pp. 388–. ISBN 978-3-540-38916-3.
- 1 2 Andrew N. Margioris; George P. Chrousos (20 April 2001). Adrenal Disorders. Springer Science & Business Media. pp. 84–. ISBN 978-1-59259-101-5.
- 1 2 Polderman KH, Gooren LJ, van der Veen EA (October 1995). "Effects of gonadal androgens and oestrogens on adrenal androgen levels". Clin. Endocrinol. (Oxf). 43 (4): 415–21. doi:10.1111/j.1365-2265.1995.tb02611.x. PMID 7586614.
- ↑ Stege R, Eriksson A, Henriksson P, Carlström K (August 1987). "Orchidectomy or oestrogen treatment in prostatic cancer: effects on serum levels of adrenal androgens and related steroids". Int. J. Androl. 10 (4): 581–7. doi:10.1111/j.1365-2605.1987.tb00357.x. PMID 2958420.
- ↑ Cox RL, Crawford ED (December 1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/S0022-5347(01)66670-9. PMID 7500443.
- 1 2 Pousette A, Carlström K, Stege R (1989). "Androgens during different modes of endocrine treatment of prostatic cancer". Urol. Res. 17 (2): 95–8. doi:10.1007/BF00262027. PMID 2734983.
- 1 2 Ottosson UB, Carlström K, Johansson BG, von Schoultz B (1986). "Estrogen induction of liver proteins and high-density lipoprotein cholesterol: comparison between estradiol valerate and ethinyl estradiol". Gynecol. Obstet. Invest. 22 (4): 198–205. doi:10.1159/000298914. PMID 3817605.
- ↑ Fotherby K (August 1996). "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception. 54 (2): 59–69. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
- ↑ Victor Gomel; Malcolm G. Munro; Timothy C. Rowe (1990). Gynecology: a practical approach. Williams & Wilkins. p. 132,134. ISBN 978-0-683-03631-2.
The synthetic estrogen, ethinyl estradiol, more commonly used in oral contraceptives, has a biological activity 100 times that of the native and conjugated substances.
- ↑ Nathaniel McConaghy (21 November 2013). Sexual Behavior: Problems and Management. Springer Science & Business Media. pp. 177–. ISBN 978-1-4899-1133-9.
Meyer et al. found that ethinyl estradiol was 75 to 100 times more potent than conjugated estrogen on the basis of the doses required to lower testosterone to the adult female range, 0.1 mg of the former and 7.5 to 10 mg of the latter being necessary.
- 1 2 Bruce Chabner; Dan Louis Longo (1996). Cancer Chemotherapy and Biotherapy: Principles and Practice. Lippincott-Raven Publishers. p. 186. ISBN 978-0-397-51418-2.
Piperazine estrone sulfate and micronized estradiol were equipotent with respect to increases in SHBG, [...] With respect to decreased FSH, [...] ethinyl estradiol was 80 to 200-fold more potent than was piperazine estrone sulfate. [...] The initial half-life of DES is 80 minutes, with a secondary half-life of 24 hours.222
- ↑ Goldzieher JW, Brody SA (1990). "Pharmacokinetics of ethinyl estradiol and mestranol". American Journal of Obstetrics and Gynecology. 163 (6 Pt 2): 2114–9. doi:10.1016/0002-9378(90)90550-Q. PMID 2256522.
- ↑ Elger W, Wyrwa R, Ahmed G, Meece F, Nair HB, Santhamma B, Killeen Z, Schneider B, Meister R, Schubert H, Nickisch K (January 2017). "Estradiol prodrugs (EP) for efficient oral estrogen treatment and abolished effects on estrogen modulated liver functions". J. Steroid Biochem. Mol. Biol. 165 (Pt B): 305–311. doi:10.1016/j.jsbmb.2016.07.008. PMID 27449818.
- 1 2 3 4 Marc A. Fritz; Leon Speroff (28 March 2012). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 753–. ISBN 978-1-4511-4847-3.
- 1 2 Notelovitz M (March 2006). "Clinical opinion: the biologic and pharmacologic principles of estrogen therapy for symptomatic menopause". MedGenMed. 8 (1): 85. PMC 1682006. PMID 16915215.
- 1 2 Goodman MP (February 2012). "Are all estrogens created equal? A review of oral vs. transdermal therapy". J Womens Health (Larchmt). 21 (2): 161–9. doi:10.1089/jwh.2011.2839. PMID 22011208.
- 1 2 Nachtigall LE, Raju U, Banerjee S, Wan L, Levitz M (2000). "Serum estradiol-binding profiles in postmenopausal women undergoing three common estrogen replacement therapies: associations with sex hormone-binding globulin, estradiol, and estrone levels". Menopause. 7 (4): 243–50. ISSN 1072-3714. PMID 10914617.
- ↑ Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R, von Schoultz B (1989). "A comparison of androgen status in patients with prostatic cancer treated with oral and/or parenteral estrogens or by orchidectomy". Prostate. 14 (2): 177–82. doi:10.1002/pros.2990140210. PMID 2523531.
- 1 2 Scarabin PY (December 2014). "Hormones and venous thromboembolism among postmenopausal women". Climacteric. 17 Suppl 2: 34–7. doi:10.3109/13697137.2014.956717. PMID 25223916.
- 1 2 Mohammed K, Abu Dabrh AM, Benkhadra K, Al Nofal A, Carranza Leon BG, Prokop LJ, Montori VM, Faubion SS, Murad MH (November 2015). "Oral vs Transdermal Estrogen Therapy and Vascular Events: A Systematic Review and Meta-Analysis". J. Clin. Endocrinol. Metab. 100 (11): 4012–20. doi:10.1210/jc.2015-2237. PMID 26544651.
- ↑ Bińkowska M (October 2014). "Menopausal hormone therapy and venous thromboembolism". Prz Menopauzalny. 13 (5): 267–72. doi:10.5114/pm.2014.46468. PMC 4520375. PMID 26327865.
- ↑ S. Campbell (6 December 2012). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. Springer Science & Business Media. pp. 395–. ISBN 978-94-011-6165-7.
- ↑ Phillips I, Shah SI, Duong T, Abel P, Langley RE (2014). "Androgen Deprivation Therapy and the Re-emergence of Parenteral Estrogen in Prostate Cancer" (PDF). Oncol Hematol Rev. 10 (1): 42–47. PMC 4052190. PMID 24932461.
- ↑ Henriksson P, Carlström K, Pousette A, Gunnarsson PO, Johansson CJ, Eriksson B, Altersgård-Brorsson AK, Nordle O, Stege R (1999). "Time for revival of estrogens in the treatment of advanced prostatic carcinoma? Pharmacokinetics, and endocrine and clinical effects, of a parenteral estrogen regimen". Prostate. 40 (2): 76–82. doi:10.1002/(sici)1097-0045(19990701)40:2<76::aid-pros2>3.0.co;2-q. PMID 10386467.
- ↑ Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- 1 2 3 Shellenberger, T. E. (1986). "Pharmacology of estrogens": 393–410. doi:10.1007/978-94-009-4145-8_36.
Ethinyl estradiol is a synthetic and comparatively potent estrogen. As a result of the alkylation in 17-C position it is not a substrate for 17β dehydrogenase, an enzyme which transforms natural estradiol-17β to the less potent estrone in target organs.
- ↑ Fruzzetti F, Trémollieres F, Bitzer J (2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecological Endocrinology : the Official Journal of the International Society of Gynecological Endocrinology. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- ↑ Sitruk-Ware R, Nath A (2011). "Metabolic effects of contraceptive steroids". Rev Endocr Metab Disord. 12 (2): 63–75. doi:10.1007/s11154-011-9182-4. PMID 21538049.
- ↑ Mueck AO, Seeger H, Rabe T (December 2010). "Hormonal contraception and risk of endometrial cancer: a systematic review". Endocr. Relat. Cancer. 17 (4): R263–71. doi:10.1677/ERC-10-0076. PMID 20870686.
- ↑ Alfred S. Wolf; H.P.G. Schneider (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 78–. ISBN 978-3-642-75101-1.
- ↑ Manfred Kaufmann; Serban-Dan Costa; Anton Scharl (27 November 2013). Die Gynäkologie. Springer-Verlag. pp. 105–. ISBN 978-3-662-11496-4.
- ↑ Mashchak CA, Lobo RA, Dozono-Takano R, Eggena P, Nakamura RM, Brenner PF, Mishell DR (November 1982). "Comparison of pharmacodynamic properties of various estrogen formulations". Am. J. Obstet. Gynecol. 144 (5): 511–8. doi:10.1016/0002-9378(82)90218-6. PMID 6291391.
- ↑ Helgason S (1982). "Estrogen replacement therapy after the menopause. Estrogenicity and metabolic effects". Acta Obstet Gynecol Scand Suppl. 107: 1–29. doi:10.3109/00016348209155333. PMID 6282033.
- ↑ Lobo RA, Nguyen HN, Eggena P, Brenner PF (February 1988). "Biologic effects of equilin sulfate in postmenopausal women". Fertil. Steril. 49 (2): 234–8. doi:10.1016/S0015-0282(16)59708-8. PMID 3338581.
- 1 2 3 http://www.ilexmedical.com/files/PDF/Estradiol_ARC.pdf
- 1 2 3 M. Notelovitz; P.A. van Keep (6 December 2012). The Climacteric in Perspective: Proceedings of the Fourth International Congress on the Menopause, held at Lake Buena Vista, Florida, October 28 – November 2, 1984. Springer Science & Business Media. pp. 397, 399. ISBN 978-94-009-4145-8.
[...] following the menopause, circulating estradiol levels decrease from a premenopausal mean of 120 pg/ml to only 13 pg/ml.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 C. Christian; B. von Schoultz (15 March 1994). Hormone Replacement Therapy: Standardized or Individually Adapted Doses?. CRC Press. pp. 9–16, 60. ISBN 978-1-85070-545-1.
The mean integrated estradiol level during a full 28-day normal cycle is around 80 pg/ml.
- ↑ Eugenio E. Müller; Robert M. MacLeod (6 December 2012). Neuroendocrine Perspectives. Springer Science & Business Media. pp. 121–. ISBN 978-1-4612-3554-5.
[...] [premenopausal] mean [estradiol] concentration of 150 pg/ml [...]
- 1 2 Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 889, 1059–1060, 2153. ISBN 978-0-7817-1750-2.
- ↑ Troisi R, Potischman N, Roberts JM, Harger G, Markovic N, Cole B, Lykins D, Siiteri P, Hoover RN (2003). "Correlation of serum hormone concentrations in maternal and umbilical cord samples". Cancer Epidemiol. Biomarkers Prev. 12 (5): 452–6. PMID 12750241.
- 1 2 Nichols KC, Schenkel L, Benson H (1984). "17 beta-estradiol for postmenopausal estrogen replacement therapy". Obstet Gynecol Surv. 39 (4): 230–45. PMID 6717863.
- 1 2 3 Dada OA, Laumas V, Landgren BM, Cekan SZ, Diczfalusy E (1978). "Effect of graded oral doses of oestradiol on circulating hormonal levels". Acta Endocrinol. 88 (4): 754–67. doi:10.1530/acta.0.0880754. PMID 581116.
- 1 2 Price TM, Blauer KL, Hansen M, Stanczy F, Lobo R, Bates W (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17β-estradiol". Am. J. Obstet. Gynecol. 89 (3): 340–345. doi:10.1016/S0029-7844(96)00513-3.
- 1 2 3 Burnier AM, Martin PL, Yen SS, Brooks P (1981). "Sublingual absorption of micronized 17beta-estradiol". Am. J. Obstet. Gynecol. 140 (2): 146–50. doi:10.1016/0002-9378(81)90101-0. PMID 6786097.
- ↑ Fiet J, Hermano M, Witte J, Villette JM, Haimart M, Gourmel B, Tabuteau F, Rouffy J, Dreux C (1982). "Post-menopausal concentrations of plasma oestradiol, oestrone, FSH and LH and of total urinary oestradiol and oestrone after a single sublingual dose of oestradiol-17 beta". Acta Endocrinol. 101 (1): 93–7. PMID 6812348.
- 1 2 Rigg LA, Milanes B, Villanueva B, Yen SS (1977). "Efficacy of intravaginal and intranasal administration of micronized estradiol-17beta". J. Clin. Endocrinol. Metab. 45 (6): 1261–4. doi:10.1210/jcem-45-6-1261. PMID 591620.
- ↑ Strecker JR, Lauritzen C, Goessens L (1979). "Plasma concentrations of unconjugated and conjugated estrogens and gonadotrophins following application of various estrogen preparations after oophorectomy and in the menopause". Maturitas. 1 (3): 183–90. PMID 228157.
- 1 2 3 Whitehead MI, Townsend PT, Kitchin Y, Dyer G, Iqbal MJ, Mansfield MD, et al. (1980). "Plasma steroid and protein hormone profiles in postmenopausal women following topical administration of oestradiol 17β". In Mauvais-Jarvis P. Percutaneous Absorption of Steroids. Academic Press. p. 231.
- ↑ Schiff I, Tulchinsky D, Ryan KJ (1977). "Vaginal absorption of estrone and 17beta-estradiol". Fertil. Steril. 28 (10): 1063–6. PMID 908445.
- 1 2 3 4 5 6 7 8 9 10 11 Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (April 1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
- 1 2 3 Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- ↑ Cynthia C. Chernecky; Barbara J. Berger (31 October 2012). Laboratory Tests and Diagnostic Procedures – E-Book. Elsevier Health Sciences. pp. 488–. ISBN 1-4557-4502-2.
- 1 2 3 4 Powers MS, Schenkel L, Darley PE, Good WR, Balestra JC, Place VA (August 1985). "Pharmacokinetics and pharmacodynamics of transdermal dosage forms of 17 beta-estradiol: comparison with conventional oral estrogens used for hormone replacement". Am. J. Obstet. Gynecol. 152 (8): 1099–106. doi:10.1016/0002-9378(85)90569-1. PMID 2992279.
- ↑ Lalit Bajaj; Stephen Berman (1 January 2011). Berman's Pediatric Decision Making. Elsevier Health Sciences. pp. 160–. ISBN 0-323-05405-6.
- ↑ Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B (December 2015). "Pharmacokinetics of the first combination 17β-estradiol/progesterone capsule in clinical development for menopausal hormone therapy". Menopause. 22 (12): 1308–16. doi:10.1097/GME.0000000000000467. PMC 4666011. PMID 25944519.
- 1 2 3 H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 61, 64. ISBN 978-1-4612-5525-3.
- ↑ Yen SS, Martin PL, Burnier AM, Czekala NM, Greaney MO, Callantine MR (March 1975). "Circulating estradiol, estrone and gonadotropin levels following the administration of orally active 17beta-estradiol in postmenopausal women". J. Clin. Endocrinol. Metab. 40 (3): 518–21. doi:10.1210/jcem-40-3-518. PMID 1117058.
- 1 2 Center for Drug Evaluation and Research. Office of Generic Drugs. Division of Bioequivalence. Application Number: 40326. Review of a Fasting Bioequivalence Study, Dissolution Data and Two Waiver Requests. Estradiol Tablets. ANDA # 40-326. Mylan Pharmaceuticals. https://www.accessdata.fda.gov/drugsatfda_docs/anda/99/40326_estradiol_bioeqr.pdf
- 1 2 3 4 5 6 Kuhnz, W.; Blode, H.; Zimmermann, H. (1993). "Pharmacokinetics of Exogenous Natural and Synthetic Estrogens and Antiestrogens". 135 / 2: 261–322. doi:10.1007/978-3-642-60107-1_15. ISSN 0171-2004.
- 1 2 Kuhnz W, Gansau C, Mahler M (September 1993). "Pharmacokinetics of estradiol, free and total estrone, in young women following single intravenous and oral administration of 17 beta-estradiol". Arzneimittelforschung. 43 (9): 966–73. PMID 8240460.
- 1 2 3 4 O'Connell MB (1995). "Pharmacokinetic and pharmacologic variation between different estrogen products". J Clin Pharmacol. 35 (9 Suppl): 18S–24S. PMID 8530713.
- 1 2 3 A. Wayne Meikle (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 380–. ISBN 978-1-59259-700-0.
- ↑ V. H. T. James; J. R. Pasqualini (22 October 2013). Hormonal Steroids: Proceedings of the Sixth International Congress on Hormonal Steroids. Elsevier Science. pp. 821–. ISBN 978-1-4831-9067-9.
- 1 2 3 4 5 6 7 8 Slater CC, Hodis HN, Mack WJ, Shoupe D, Paulson RJ, Stanczyk FZ (2001). "Markedly elevated levels of estrone sulfate after long-term oral, but not transdermal, administration of estradiol in postmenopausal women". Menopause. 8 (3): 200–3. PMID 11355042.
- ↑ Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, Dickler MN, Silverman P, Fleming GF, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Siegel BA (August 2009). "Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study". JAMA. 302 (7): 774–80. doi:10.1001/jama.2009.1204. PMC 3460383. PMID 19690310.
- 1 2 3 4 5 6 7 Selby P, McGarrigle HH, Peacock M (March 1989). "Comparison of the effects of oral and transdermal oestradiol administration on oestrogen metabolism, protein synthesis, gonadotrophin release, bone turnover and climacteric symptoms in postmenopausal women". Clin. Endocrinol. (Oxf). 30 (3): 241–9. doi:10.1111/j.1365-2265.1989.tb02232.x. PMID 2512035.
- 1 2 Rebekah Wang-Cheng; Joan M. Neuner; Vanessa M. Barnabei (2007). Menopause. ACP Press. pp. 91–. ISBN 978-1-930513-83-9.
- 1 2 John J.B. Anderson; Sanford C. Garner (24 October 1995). Calcium and Phosphorus in Health and Disease. CRC Press. pp. 215–216. ISBN 978-0-8493-7845-4.
- ↑ Eef Hogervorst (24 September 2009). Hormones, Cognition and Dementia: State of the Art and Emergent Therapeutic Strategies. Cambridge University Press. pp. 82–. ISBN 978-0-521-89937-6.
- ↑ Wright JV (December 2005). "Bio-identical steroid hormone replacement: selected observations from 23 years of clinical and laboratory practice". Ann. N. Y. Acad. Sci. 1057: 506–24. Bibcode:2005NYASA1057..506W. doi:10.1196/annals.1356.039. PMID 16399916.
- 1 2 Friel PN, Hinchcliffe C, Wright JV (March 2005). "Hormone replacement with estradiol: conventional oral doses result in excessive exposure to estrone". Altern Med Rev. 10 (1): 36–41. PMID 15771561.
- ↑ Lobo RA (March 1987). "Absorption and metabolic effects of different types of estrogens and progestogens". Obstet. Gynecol. Clin. North Am. 14 (1): 143–67. PMID 3306517.
- 1 2 Laura Marie Borgelt (2010). Women's Health Across the Lifespan: A Pharmacotherapeutic Approach. ASHP. pp. 257–. ISBN 978-1-58528-194-7.
- ↑ Jose Russo; Irma H. Russo (28 June 2011). Molecular Basis of Breast Cancer: Prevention and Treatment. Springer Science & Business Media. pp. 92–. ISBN 978-3-642-18736-0.
- ↑ Sue E. Huether; Kathryn L. McCance (27 December 2013). Understanding Pathophysiology. Elsevier Health Sciences. pp. 845–. ISBN 978-0-323-29343-3.
- ↑ Ruggiero RJ, Likis FE (2002). "Estrogen: physiology, pharmacology, and formulations for replacement therapy". Journal of Midwifery & Women's Health. 47 (3): 130–8. doi:10.1016/s1526-9523(02)00233-7. PMID 12071379.
- 1 2 Fåhraeus L, Larsson-Cohn U (December 1982). "Oestrogens, gonadotrophins and SHBG during oral and cutaneous administration of oestradiol-17 beta to menopausal women". Acta Endocrinol. 101 (4): 592–6. doi:10.1530/acta.0.1010592. PMID 6818806.
- ↑ Kloosterboer, HJ; Schoonen, WG; Verheul, HA (11 April 2008). "Proliferation of Breast Cells by Steroid Hormones and Their Metabolites". In Pasqualini, Jorge R. Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 343–366. ISBN 978-1-4200-5873-4.
- ↑ Sasson S, Notides AC (July 1983). "Estriol and estrone interaction with the estrogen receptor. II. Estriol and estrone-induced inhibition of the cooperative binding of [3H]estradiol to the estrogen receptor". J. Biol. Chem. 258 (13): 8118–22. PMID 6863280.
- ↑ Lundström E, Conner P, Naessén S, Löfgren L, Carlström K, Söderqvist G (2015). "Estrone - a partial estradiol antagonist in the normal breast". Gynecol. Endocrinol. 31 (9): 747–9. doi:10.3109/09513590.2015.1062866. PMID 26190536.
- ↑ Martel C, Rhéaume E, Takahashi M, Trudel C, Couët J, Luu-The V, Simard J, Labrie F (March 1992). "Distribution of 17 beta-hydroxysteroid dehydrogenase gene expression and activity in rat and human tissues". J. Steroid Biochem. Mol. Biol. 41 (3–8): 597–603. PMID 1314080.
- 1 2 Miki Y, Nakata T, Suzuki T, Darnel AD, Moriya T, Kaneko C, Hidaka K, Shiotsu Y, Kusaka H, Sasano H (December 2002). "Systemic distribution of steroid sulfatase and estrogen sulfotransferase in human adult and fetal tissues". J. Clin. Endocrinol. Metab. 87 (12): 5760–8. doi:10.1210/jc.2002-020670. PMID 12466383.
- ↑ Laura Marie Borgelt (2010). Women's Health Across the Lifespan: A Pharmacotherapeutic Approach. ASHP. pp. 256–. ISBN 978-1-58528-194-7.
- 1 2 Rezvanpour A, Don-Wauchope AC (March 2017). "Clinical implications of estrone sulfate measurement in laboratory medicine". Crit Rev Clin Lab Sci. 54 (2): 73–86. doi:10.1080/10408363.2016.1252310. PMID 27960570.
- ↑ Crandall CJ, Guan M, Laughlin GA, Ursin GA, Stanczyk FZ, Ingles SA, Barrett-Connor E, Greendale GA (July 2008). "Increases in serum estrone sulfate level are associated with increased mammographic density during menopausal hormone therapy". Cancer Epidemiol. Biomarkers Prev. 17 (7): 1674–81. doi:10.1158/1055-9965.EPI-07-2779. PMC 2745228. PMID 18628419.
- 1 2 Jorge R. Pasqualini (17 July 2002). Breast Cancer: Prognosis, Treatment, and Prevention. CRC Press. pp. 195–. ISBN 978-0-203-90924-9.
- ↑ James MR, Skaar TC, Lee RY, MacPherson A, Zwiebel JA, Ahluwalia BS, Ampy F, Clarke R (April 2001). "Constitutive expression of the steroid sulfatase gene supports the growth of MCF-7 human breast cancer cells in vitro and in vivo". Endocrinology. 142 (4): 1497–505. doi:10.1210/endo.142.4.8091. PMID 11250930.
- 1 2 Alkjaersig N, Fletcher AP, de Ziegler D, Steingold KA, Meldrum DR, Judd HL (1988). "Blood coagulation in postmenopausal women given estrogen treatment: comparison of transdermal and oral administration". J. Lab. Clin. Med. 111 (2): 224–8. PMID 2448408.
- 1 2 Weissberger AJ, Ho KK, Lazarus L (1991). "Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and GH-binding protein in postmenopausal women". J. Clin. Endocrinol. Metab. 72 (2): 374–81. doi:10.1210/jcem-72-2-374. PMID 1991807.
- 1 2 Sonnet E, Lacut K, Roudaut N, Mottier D, Kerlan V, Oger E (2007). "Effects of the route of oestrogen administration on IGF-1 and IGFBP-3 in healthy postmenopausal women: results from a randomized placebo-controlled study". Clin. Endocrinol. 66 (5): 626–31. doi:10.1111/j.1365-2265.2007.02783.x. PMID 17492948.
- 1 2 Nugent AG, Leung KC, Sullivan D, Reutens AT, Ho KK (2003). "Modulation by progestogens of the effects of oestrogen on hepatic endocrine function in postmenopausal women". Clin. Endocrinol. 59 (6): 690–8. doi:10.1046/j.1365-2265.2003.01907.x. PMID 14974909.
- 1 2 Isotton, A. L.; Wender, M. C. O.; Casagrande, A.; Rollin, G.; Czepielewski, M. A. (2011). "Effects of oral and transdermal estrogen on IGF1, IGFBP3, IGFBP1, serum lipids, and glucose in patients with hypopituitarism during GH treatment: a randomized study" (PDF). European Journal of Endocrinology. 166 (2): 207–213. doi:10.1530/EJE-11-0560. ISSN 0804-4643. PMID 22108915.
- 1 2 Jasonni VM, Bulletti C, Naldi S, Ciotti P, Di Cosmo D, Lazzaretto R, et al. (1988). "Biological and endocrine aspects of transdermal 17 beta-oestradiol administration in post-menopausal women". Maturitas. 10 (4): 263–70. doi:10.1016/0378-5122(88)90062-x. PMID 3226336.
- ↑ Dansuk R, Unal O, Karageyim Y, Esim E, Turan C (2004). "Evaluation of the effect of tibolone and transdermal estradiol on triglyceride level in hypertriglyceridemic and normotriglyceridemic postmenopausal women". Gynecol. Endocrinol. 18 (5): 233–9. doi:10.1080/09513590410001715199. PMID 15346658.
- ↑ Santoro N, Worsley R, Miller KK, Parish SJ, Davis SR (March 2016). "Role of Estrogens and Estrogen-Like Compounds in Female Sexual Function and Dysfunction". J Sex Med. 13 (3): 305–16. doi:10.1016/j.jsxm.2015.11.015. PMID 26944462.
- 1 2 3 4 5 6 7 8 9 10 Alfred S. Wolf; H.P.G. Schneider (12 March 2013). Östrogene in Diagnostik und Therapie. Springer-Verlag. pp. 79, 81. ISBN 978-3-642-75101-1.
- ↑ Wren BG, Day RO, McLachlan AJ, Williams KM (June 2003). "Pharmacokinetics of estradiol, progesterone, testosterone and dehydroepiandrosterone after transbuccal administration to postmenopausal women". Climacteric. 6 (2): 104–11. doi:10.1080/cmt.6.2.104.111. PMID 12841880.
- ↑ Gass MS, Rebar RW, Cuffie-Jackson C, Cedars MI, Lobo RA, Shoupe D, Judd HL, Buyalos RP, Clisham PR (October 2004). "A short study in the treatment of hot flashes with buccal administration of 17-beta estradiol". Maturitas. 49 (2): 140–7. doi:10.1016/j.maturitas.2003.12.004. PMID 15474758.
- 1 2 Price TM, Blauer KL, Hansen M, Stanczyk F, Lobo R, Bates GW (1997). "Single-dose pharmacokinetics of sublingual versus oral administration of micronized 17 beta-estradiol". Obstet Gynecol. 89 (3): 340–5. doi:10.1016/S0029-7844(96)00513-3. PMID 9052581.
- ↑ Casper RF, Yen SS (1981). "Rapid absorption of micronized estradiol-17 beta following sublingual administration". Obstet Gynecol. 57 (1): 62–4. PMID 7454177.
- ↑ Pines A, Averbuch M, Fisman EZ, Rosano GM (1999). "The acute effects of sublingual 17beta-estradiol on the cardiovascular system". Maturitas. 33 (1): 81–5. doi:10.1016/s0378-5122(99)00036-5. PMID 10585176.
- ↑ Dooley, Mukta; Spencer, Caroline M.; Ormrod, Douglas (2001). "Estradiol-Intranasal". Drugs. 61 (15): 2243–2262. doi:10.2165/00003495-200161150-00012. ISSN 0012-6667.
- ↑ Lopes P, Rozenberg S, Graaf J, Fernandez-Villoria E, Marianowski L (2001). "Aerodiol versus the transdermal route: perspectives for patient preference". Maturitas. 38 Suppl 1: S31–9. PMID 11390122.
- ↑ Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 173–. ISBN 978-0-08-093292-7.
- ↑ Janice Ryden; Paul D. Blumenthal (2002). Practical Gynecology: A Guide for the Primary Care Physician. ACP Press. pp. 436–. ISBN 978-0-943126-94-4.
- ↑ Sahin FK, Koken G, Cosar E, Arioz DT, Degirmenci B, Albayrak R, Acar M (2008). "Effect of Aerodiol administration on ocular arteries in postmenopausal women". Gynecol. Endocrinol. 24 (4): 173–7. doi:10.1080/09513590701807431. PMID 18382901.
300 μg 17β-estradiol (Aerodiol®; Servier, Chambrayles-Tours, France) was administered via the nasal route by a gynecologist. This product is unavailable after March 31, 2007 because its manufacturing and marketing are being discontinued.
- ↑ http://www.netdoctor.co.uk/medicines/a8266/aerodiol-nasal-spray-discontinued-in-the-uk-december-2006/
- 1 2 https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20323S23lbl.pdf
- 1 2 https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020538s035lbl.pdf
- 1 2 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d28bec8f-762e-4f05-a20d-96a42970d6a7
- 1 2 https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020375s034lbl.pdf
- 1 2 https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020375s035lbl.pdf
- 1 2 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e7e6da3b-8485-1382-61c9-e9b369018b98
- 1 2 3 4 5 6 7 8 9 10 11 "EstroGel® 0.06% (Estradiol Gel) for Topical Use FDA Label" (PDF). Food and Drug Administration. 2017. Retrieved 22 July 2018.
- 1 2 3 Vinod P. Shah; Howard I. Maibach (29 June 2013). Topical Drug Bioavailability, Bioequivalence, and Penetration. Springer Science & Business Media. pp. 47–50. ISBN 978-1-4899-1262-6.
- 1 2 Comprehensive Toxicology. Elsevier Science. 1 December 2017. pp. 2–. ISBN 978-0-08-100612-2.
- 1 2 3 4 5 6 7 James M. Rippe (15 March 2013). Lifestyle Medicine, Second Edition. CRC Press. pp. 279–. ISBN 978-1-4398-4542-4.
- 1 2 "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 26 July 2018.
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020655
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020655s020lbl.pdf
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020375
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021885
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021258s011lbl.pdf
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020870
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/020870s023lbl.pdf
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020847
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/1998/20847lblmd.pdf
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=019081
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019081s043lbl.pdf
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=201675
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=075182
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020417
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021674
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=203752
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/203752s009lbl.pdf
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=021167
- ↑ https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=020538
- ↑ Manfred Kaufmann; Serban-Dan Costa; Anton Scharl (27 November 2013). Die Gynäkologie. Springer-Verlag. pp. 106–. ISBN 978-3-662-11496-4.
- ↑ Hall G, Blombäck M, Landgren BM, Bremme K (2002). "Effects of vaginally administered high estradiol doses on hormonal pharmacokinetics and hemostasis in postmenopausal women". Fertil. Steril. 78 (6): 1172–7. doi:10.1016/s0015-0282(02)04285-1. PMID 12477507.
- 1 2 3 Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- 1 2 3 4 5 6 7 8 9 Lauritzen C (December 1986). "Die Behandlung der klimakterischen Beschwerden durch vaginale, rektale und transdermale Ostrogensubstitution" [Treatment of disorders of the climacteric by vaginal, rectal and transdermal estrogen substitution]. Gynakologe (in German). 19 (4): 248–53. ISSN 0017-5994. PMID 3817597.
- ↑ Göretzlehner G (1989). "Wirkungsstärke unterschiedlicher Estrogene in Abhängigkeit vom Applikationsmodus" [Efficacy of different estrogens as subject to mode of application]. Zentralbl Gynakol (in German). 111 (16): 1093–100. ISSN 0044-4197. PMID 2683509.
- 1 2 Sambuelli M (1953). "Somministrazione degli estrogeni per via rettale nel carcinoma prostatico" [Rectal administration of estrogens in prostate carcinoma]. Minerva Urol (in Undetermined). 5 (1): 28–32. ISSN 0026-4989. PMID 13063334.
Subject: Estrogens/Therapeutic Use; Prostate/Neoplasms; Administration, Rectal; Contraceptive Agents, Female; Estradiol Congeners; Prostatic Neoplasms; Estrogens -- Therapeutic Use
- ↑ Nizza M, Giardinelli M (August 1954). "Studio dell'attività degli estrogeni naturali e sintetici somministrati per via rettale mediante il controllo degli strisci vaginali" [Activity of natural and synthetic estrogens administered by the rectal route: study by vaginal smears]. Minerva Ginecol (in Italian). 6 (15): 548–51. ISSN 0026-4784. PMID 13203215.
- 1 2 Moran DJ, McGarrigle HH, Lachelin GC (January 1994). "Maternal plasma progesterone levels fall after rectal administration of estriol". J. Clin. Endocrinol. Metab. 78 (1): 70–2. doi:10.1210/jcem.78.1.8288717. PMID 8288717.
- ↑ Anthony W. Norman; Gerald Litwack (23 October 1997). Hormones. Academic Press. pp. 398–. ISBN 978-0-08-053413-8.
- ↑ Asim Kurjak; Frank A. Chervenak (25 September 2006). Textbook of Perinatal Medicine, Second Edition. CRC Press. pp. 699–. ISBN 978-1-4398-1469-7.
- ↑ Michael F Greene; Robert K. Creasy; Robert Resnik; Jay D. Iams; Charles J. Lockwood; Thomas Moore (25 November 2008). Creasy and Resnik's Maternal-Fetal Medicine: Principles and Practice E-Book. Elsevier Health Sciences. pp. 115–. ISBN 1-4377-2135-4.
- 1 2 3 4 Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- 1 2 Nagrath Arun; Malhotra Narendra; Seth Shikha (15 December 2012). Progress in Obstetrics and Gynecology—3. Jaypee Brothers Medical Publishers Pvt. Ltd. pp. 416–418. ISBN 978-93-5090-575-3.
- ↑ Ulrich U, Pfeifer T, Lauritzen C (1994). "Rapid increase in lumbar spine bone density in osteopenic women by high-dose intramuscular estrogen-progestogen injections. A preliminary report". Horm. Metab. Res. 26 (9): 428–31. doi:10.1055/s-2007-1001723. PMID 7835827.
- ↑ Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. PMID 10230677.
- 1 2 http://pharmanovia.com/product/estradurin/
- ↑ https://www.drugs.com/international/polyestradiol-phosphate.html
- 1 2 3 4 "Juvenum E (estradiol) - Medicamentos PLM". Medicamentos PLM. Archived from the original on 2018-09-17. Retrieved 2018-09-17.
- ↑ https://www.drugs.com/international/juvenum-e.html
- 1 2 Unger CA (December 2016). "Hormone therapy for transgender patients". Transl Androl Urol. 5 (6): 877–884. doi:10.21037/tau.2016.09.04. PMC 5182227. PMID 28078219.
- ↑ Al-Futaisi AM, Al-Zakwani IS, Almahrezi AM, Morris D (December 2006). "Subcutaneous administration of testosterone. A pilot study report" (PDF). Saudi Med J. 27 (12): 1843–6. PMID 17143361.
- ↑ Olson J, Schrager SM, Clark LF, Dunlap SL, Belzer M (September 2014). "Subcutaneous Testosterone: An Effective Delivery Mechanism for Masculinizing Young Transgender Men". LGBT Health. 1 (3): 165–7. doi:10.1089/lgbt.2014.0018. PMID 26789709.
- ↑ Spratt DI, Stewart II, Savage C, Craig W, Spack NP, Chandler DW, Spratt LV, Eimicke T, Olshan JS (July 2017). "Subcutaneous Injection of Testosterone Is an Effective and Preferred Alternative to Intramuscular Injection: Demonstration in Female-to-Male Transgender Patients". J. Clin. Endocrinol. Metab. 102 (7): 2349–2355. doi:10.1210/jc.2017-00359. PMID 28379417.
- ↑ McFarland J, Craig W, Clarke NJ, Spratt DI (August 2017). "Serum Testosterone Concentrations Remain Stable Between Injections in Patients Receiving Subcutaneous Testosterone". J Endocr Soc. 1 (8): 1095–1103. doi:10.1210/js.2017-00148. PMC 5686655. PMID 29264562.
- ↑ Wilson DM, Kiang TK, Ensom MH (March 2018). "Pharmacokinetics, safety, and patient acceptability of subcutaneous versus intramuscular testosterone injection for gender-affirming therapy: A pilot study". Am J Health Syst Pharm. 75 (6): 351–358. doi:10.2146/ajhp170160. PMID 29367424.
- ↑ Singh GK, Turner L, Desai R, Jimenez M, Handelsman DJ (July 2014). "Pharmacokinetic-pharmacodynamic study of subcutaneous injection of depot nandrolone decanoate using dried blood spots sampling coupled with ultrapressure liquid chromatography tandem mass spectrometry assays". J. Clin. Endocrinol. Metab. 99 (7): 2592–8. doi:10.1210/jc.2014-1243. PMID 24684468.
- 1 2 Chan VO, Colville J, Persaud T, Buckley O, Hamilton S, Torreggiani WC (June 2006). "Intramuscular injections into the buttocks: are they truly intramuscular?". Eur J Radiol. 58 (3): 480–4. doi:10.1016/j.ejrad.2006.01.008. PMID 16495027.
- 1 2 Palma S, Strohfus P (November 2013). "Are IM injections IM in obese and overweight females? A study in injection technique". Appl Nurs Res. 26 (4): e1–4. doi:10.1016/j.apnr.2013.09.002. PMID 24156877.
- 1 2 Sherif, Katherine (2013). "The Significance of Hormone Routes of Administration": 57–61. doi:10.1007/978-1-4614-6268-2_7.
- 1 2 3 4 Ian M. Symonds; Sabaratnam Arulkumaran (3 August 2013). Essential Obstetrics and Gynaecology E-Book. Elsevier Health Sciences. pp. 258–. ISBN 978-0-7020-5475-4.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Leo Jr. Plouffe; Veronica A. Ravnikar; Leon Speroff; Nelson B. Watts (6 December 2012). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 271–. ISBN 978-1-4612-4330-4.
- 1 2 Martin Birkhauser; David Barlow; Morris Notelovitz; Margaret Rees (12 August 2005). Health Plan for the Adult Woman: Management Handbook. CRC Press. pp. 27–. ISBN 978-0-203-49009-9.
- ↑ Pinkerton JV (December 2014). "What are the concerns about custom-compounded "bioidentical" hormone therapy?". Menopause. 21 (12): 1298–300. doi:10.1097/GME.0000000000000376. PMID 25387347.
- ↑ Pinkerton JV, Pickar JH (February 2016). "Update on medical and regulatory issues pertaining to compounded and FDA-approved drugs, including hormone therapy". Menopause. 23 (2): 215–23. doi:10.1097/GME.0000000000000523. PMC 4927324. PMID 26418479.
- ↑ Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (April 2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". J. Clin. Endocrinol. Metab. 101 (4): 1318–43. doi:10.1210/jc.2016-1271. PMID 27032319.
- ↑ Alex Khot; Andrew Polmear (18 November 2011). Practical General Practice E-Book: Guidelines for Effective Clinical Management. Elsevier Health Sciences. pp. 338–. ISBN 0-7020-4909-3.
- ↑ Kronenberg F (1990). "Hot flashes: epidemiology and physiology". Ann. N. Y. Acad. Sci. 592: 52–86, discussion 123–33. doi:10.1111/j.1749-6632.1990.tb30316.x. PMID 2197954.
- ↑ Buchsbaum HJ, ed. (2012). The Menopause (Clinical Perspectives in Obstetrics and Gynecology). New York, NY: Springer Science & Business Media. p. 64. ISBN 9781461255253.
- ↑ Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric : the Journal of the International Menopause Society. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ "EC 2.4.1.17 – glucuronosyltransferase and Organism(s) Homo sapiens". BRENDA. Technische Universität Braunschweig. January 2018. Retrieved 10 August 2018.
Further reading
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens I: Physiology and Mechanisms of Action of Estrogens and Antiestrogens. Springer Science & Business Media. ISBN 978-3-642-58616-3.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. ISBN 978-3-642-60107-1.
- Fruzzetti F, Trémollieres F, Bitzer J (2012). "An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest". Gynecol. Endocrinol. 28 (5): 400–8. doi:10.3109/09513590.2012.662547. PMC 3399636. PMID 22468839.
- Stanczyk FZ, Archer DF, Bhavnani BR (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–27. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
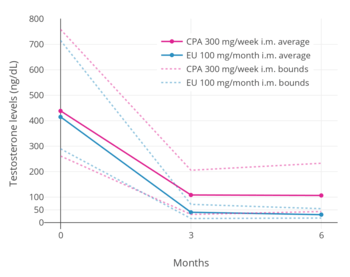
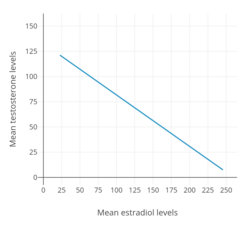


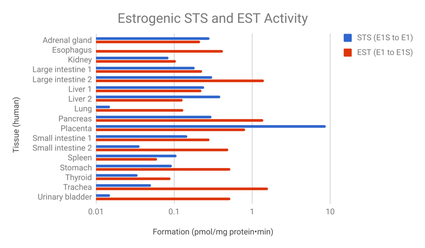
_in_postmenopausal_women.png)
_in_postmenopausal_women.png)