Ovary
| Ovary | |
|---|---|
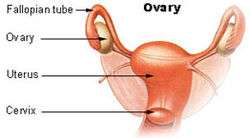 The ovaries form part of the female reproductive system, and attach to the fallopian tubes | |
 Blood supply of the human female reproductive organs. The left ovary is the oval shaped structure visible above the label "ovarian arteries". | |
| Details | |
| System | Female reproductive system |
| Artery | ovarian artery, uterine artery |
| Vein | ovarian vein |
| Nerve | ovarian plexus |
| Lymph | Paraaortic lymph node |
| Identifiers | |
| Latin | ovarium |
| MeSH | D010053 |
| TA | A09.1.01.001 |
| FMA | 7209 |
| Anatomical terminology | |
The ovary is an organ found in the female reproductive system that produces an ovum. When released, this travels down the fallopian tube into the uterus, where it may become fertilised by a sperm. There is an ovary (from Latin ovarium, meaning 'egg, nut') found on the left and right sides of the body. The ovaries also secrete hormones that play a role in the menstrual cycle and fertility. The ovary progresses through many stages beginning in the prenatal period through menopause. It is also an endocrine gland because of the various hormones that it secretes.[1]
Structure
The ovaries are considered the female gonads.[2] Each ovary is whitish in color and located alongside the lateral wall of the uterus in a region called the ovarian fossa. The ovarian fossa is the region that is bounded by the external iliac artery and in front of the ureter and the internal iliac artery. This area is about 4 cm x 3 cm x 2 cm in size.[3][4] The ovaries are surrounded by a capsule, and have an outer cortex and an inner medulla.[4]
Usually, ovulation occurs in one of the two ovaries releasing an egg each menstrual cycle; however, if there was a case where one ovary was absent or dysfunctional then the other ovary would continue providing eggs to be released without any changes in cycle length or frequency.
The side of the ovary closest to the fallopian tube is connected to it by infundibulopelvic ligament,[3] and the other side points downwards attached to the uterus via the ovarian ligament.
Other structures and tissues of the ovaries include the hilum.
Ligaments
The ovaries lie within the pelvic cavity, on either side of the uterus, to which they are attached via a fibrous cord called the ovarian ligament. The ovaries are uncovered in the peritoneal cavity but are tethered to the body wall via the suspensory ligament of the ovary which is a posterior extension of the broad ligament of the uterus. The part of the broad ligament of the uterus that covers the ovary is known as the mesovarium.[4]
The ovarian pedicle is made up part of the fallopian tube, mesovarium, ovarian ligament, and ovarian blood vessels.[5]
Microanatomy
The surface of the ovaries is covered with membrane consisting of a lining of simple cuboidal-to-columnar shaped mesothelium.[6]
The outermost layer is called the germinal epithelium.
The outer layer is the ovarian cortex, consisting of ovarian follicles and stroma in between them. Included in the follicles are the cumulus oophorus, membrana granulosa (and the granulosa cells inside it), corona radiata, zona pellucida, and primary oocyte. Theca of follicle, antrum and liquor folliculi are also contained in the follicle. Also in the cortex is the corpus luteum derived from the follicles. The innermost layer is the ovarian medulla.[7] It can be hard to distinguish between the cortex and medulla, but follicles are usually not found in the medulla.
Follicular cells flat epithelial cells that originate from surface epithelium covering the ovary, are surrounded by Granulosa cells - that have changed from flat to cuboidal and proliferated to produce a stratified epithelium
Other
The ovary also contains blood vessels and lymphatics.[9]
Function
At puberty, the ovary begins to secrete increasing levels of hormones. Secondary sex characteristics begin to develop in response to the hormones. The ability to produce eggs and reproduce develops. The ovary changes structure and function beginning at puberty.[1]
Gamete production
The ovaries are the site of production and periodical release of egg cells, the female gametes. In the ovaries, the developing egg cells (or oocytes) mature in the fluid-filled follicles. Typically, only one oocyte develops at a time, but others can also mature simultaneously. Follicles are composed of different types and number of cells according to the stage of their maturation, and their size is indicative of the stage of oocyte development.[10]:833
When the oocyte finishes its maturation in the ovary, a surge of luteinizing hormone secreted by the pituitary gland stimulates the release of the oocyte through the rupture of the follicle, a process called ovulation.[11] The follicle remains functional and reorganizes into a corpus luteum, which secretes progesterone in order to prepare the uterus for an eventual implantation of the embryo.[10]:839
Hormone secretion
At maturity, ovaries secrete estrogen, testosterone,[12][13] inhibin, and progesterone.[14][15][1] In women, fifty percent of testosterone is produced by the ovaries and adrenal glands and released directly into the blood stream.[16] Estrogen is responsible for the appearance of secondary sex characteristics for females at puberty and for the maturation and maintenance of the reproductive organs in their mature functional state. Progesterone prepares the uterus for pregnancy, and the mammary glands for lactation. Progesterone functions with estrogen by promoting menstrual cycle changes in the endometrium.
Ovarian aging
As women age, they experience a decline in reproductive performance leading to menopause. This decline is tied to a decline in the number of ovarian follicles. Although about 1 million oocytes are present at birth in the human ovary, only about 500 (about 0.05%) of these ovulate, and the rest are wasted. The decline in ovarian reserve appears to occur at a constantly increasing rate with age,[17] and leads to nearly complete exhaustion of the reserve by about age 52. As ovarian reserve and fertility decline with age, there is also a parallel increase in pregnancy failure and meiotic errors resulting in chromosomally abnormal conceptions.
Women with an inherited mutation in the DNA repair gene BRCA1 undergo menopause prematurely,[18] suggesting that naturally occurring DNA damages in oocytes are repaired less efficiently in these women, and this inefficiency leads to early reproductive failure. The BRCA1 protein plays a key role in a type of DNA repair termed homologous recombinational repair that is the only known cellular process that can accurately repair DNA double-strand breaks. Titus et al.[19] showed that DNA double-strand breaks accumulate with age in humans and mice in primordial follicles. Primordial follicles contain oocytes that are at an intermediate (prophase I) stage of meiosis. Meiosis is the general process in eukaryotic organisms by which germ cells are formed, and it is likely an adaptation for removing DNA damages, especially double-strand breaks, from germ line DNA.[20] (see Meiosis and Origin and function of meiosis). Homologous recombinational repair is especially promoted during meiosis. Titus et al.[19] also found that expression of 4 key genes necessary for homologous recombinational repair of DNA double-strand breaks (BRCA1, MRE11, RAD51 and ATM) decline with age in the oocytes of humans and mice. They hypothesized that DNA double-strand break repair is vital for the maintenance of oocyte reserve and that a decline in efficiency of repair with age plays a key role in ovarian aging.
Clinical significance
Ovarian diseases can be classified as endocrine disorders or as a disorders of the reproductive system.
If the egg fails to release from the follicle in the ovary an ovarian cyst may form. Small ovarian cysts are common in healthy women. Some women have more follicles than usual (polycystic ovary syndrome), which inhibits the follicles to grow normally and this will cause cycle irregularities.
| Notes | Ref(s) | |
|---|---|---|
| Ovarian neoplasms | ||
| Germ cell tumor | Seen most often in young women or adolescent girls Other germ cell tumors are: Endodermal sinus tumor and teratoma, |
[21] |
| Ovarian cancer | includes ovarian epithelial cancer | [22][23][24] |
| Luteoma | [25] | |
| Ovaritis | syn. oophoritis | [15] |
| Ovarian remnant syndrome | [15] | |
| Hypogonadism | ||
| Hyperthecosis | ||
| Ovarian torsion | ||
| Ovarian apoplexy (rupture) | ||
| Premature ovarian failure | ||
| Anovulation | ||
| Follicular cyst of ovary | ||
| Corpus luteum cyst | ||
| Theca lutein cyst | ||
| Chocolate cyst | ||
| Ovarian germ cell tumors | benign | [26] |
| Dysgerminoma | ||
| Choriocarcinoma | ||
| Yolk sac tumor | ||
| Teratoma | ||
| Ovarian serous cystadenoma | ||
| Serous cystadenocarcinoma | ||
| Mucinous cystadenoma | ||
| Mucinous cystadenocarcinoma | ||
| Brenner tumor | ||
| Granulosa cell tumor | ||
| Krukenberg tumor |
Society and culture
Cryopreservation
Cryopreservation of ovarian tissue, often called ovarian tissue cryopreservation, is of interest to women who want to preserve their reproductive function beyond the natural limit, or whose reproductive potential is threatened by cancer therapy,[27] for example in hematologic malignancies or breast cancer.[28] The procedure is to take a part of the ovary and carry out slow freezing before storing it in liquid nitrogen whilst therapy is undertaken. Tissue can then be thawed and implanted near the fallopian, either orthotopic (on the natural location) or heterotopic (on the abdominal wall),[28] where it starts to produce new eggs, allowing normal conception to take place.[29] A study of 60 procedures concluded that ovarian tissue harvesting appears to be safe.[28] The ovarian tissue may also be transplanted into mice that are immunocompromised (SCID mice) to avoid graft rejection, and tissue can be harvested later when mature follicles have developed.[30]
Other animals
.tif.jpg)
Birds have only one functional ovary (the left), while the other remains vestigial. Ovaries in females are analogous to testes in males, in that they are both gonads and endocrine glands. Ovaries of some kind are found in the female reproductive system of many animals that employ sexual reproduction, including invertebrates. However, they develop in a very different way in most invertebrates than they do in vertebrates, and are not truly homologous.[31]
Many of the features found in human ovaries are common to all vertebrates, including the presence of follicular cells, tunica albuginea, and so on. However, many species produce a far greater number of eggs during their lifetime than do humans, so that, in fish and amphibians, there may be hundreds, or even millions of fertile eggs present in the ovary at any given time. In these species, fresh eggs may be developing from the germinal epithelium throughout life. Corpora lutea are found only in mammals, and in some elasmobranch fish; in other species, the remnants of the follicle are quickly resorbed by the ovary. In birds, reptiles, and monotremes, the egg is relatively large, filling the follicle, and distorting the shape of the ovary at maturity.[31]
Amphibians and reptiles have no ovarian medulla; the central part of the ovary is a hollow, lymph-filled space.[32]
The ovary of teleosts is also often hollow, but in this case, the eggs are shed into the cavity, which opens into the oviduct.[31] Certain nematodes of the genus Philometra are parasitic in the ovary of marine fishes and can be spectacular, with females as long as 40 cm, coiled in the ovary of a fish half this length.[33] Although most normal female vertebrates have two ovaries, this is not the case in all species. In most birds and in platypuses, the right ovary never matures, so that only the left is functional. (Exceptions include the kiwi and some, but not all raptors, in which both ovaries persist.[34][35]) In some elasmobranchs, only the right ovary develops fully. In the primitive jawless fish, and some teleosts, there is only one ovary, formed by the fusion of the paired organs in the embryo.[31]
Additional images
- Left Ovary
 Ovaries
Ovaries
See also
References
- 1 2 3 Colvin, Caroline Wingo; Abdullatif, Hussein (2013-01-01). "Anatomy of female puberty: The clinical relevance of developmental changes in the reproductive system". Clinical Anatomy. 26 (1): 115–129. doi:10.1002/ca.22164. ISSN 1098-2353.
- ↑ "Dorlands Medical Dictionary". www.mercksource.com. Retrieved 2017-11-20.
- 1 2 Daftary, Shirish; Chakravarti, Sudip (2011). Manual of Obstetrics, 3rd Edition. Elsevier. pp. 1-16. ISBN 9788131225561.
- 1 2 3 Williams gynecology. Hoffman, Barbara L., Williams, J. Whitridge (John Whitridge), 1866-1931. (2nd ed.). New York: McGraw-Hill Medical. 2012. ISBN 9780071716727. OCLC 779244257.
- ↑ Baskett, Thomas F.; Calder, Andrew A.; Arulkumaran, Sabaratnam (2014). Munro Kerr's Operative Obstetrics E-Book. Elsevier Health Sciences. p. 268. ISBN 9780702052484.
- ↑ "Southern Illinois University School of Medicine". www.siumed.edu. Retrieved 2017-11-20.
- ↑ "Foundational Model of Anatomy". xiphoid.biostr.washington.edu. Structural Informatics Group at the University of Washington. Retrieved 2017-11-20.
- ↑ Langman's Medical Embryology, Lippincott Williams & Wilkins, 10th ed, 2006
- ↑ Brown, H. M.; Russell, D. L. (2013). "Blood and lymphatic vasculature in the ovary: Development, function and disease". Human Reproduction Update. 20: 29–39. doi:10.1093/humupd/dmt049. PMID 24097804.
- 1 2 Ross M, Pawlina W (2011). Histology: A Text and Atlas (6th ed.). Lippincott Williams & Wilkins. ISBN 978-0-7817-7200-6.
- ↑ Melmed, S; Polonsky, KS; Larsen, PR; Kronenberg, HM (2011). Williams Textbook of Endocrinology (12th ed.). Saunders. p. 595. ISBN 978-1437703245.
- ↑ "Normal Testosterone and Estrogen Levels in Women". WebMD. Retrieved 2017-11-19.
- ↑ "Testosterone: MedlinePlus Medical Encyclopedia". www.nlm.nih.gov. Retrieved 2017-11-19.
- ↑ Marieb, Elaine (2013). Anatomy & physiology. Benjamin-Cummings. p. 903. ISBN 9780321887603.
- 1 2 3 Venes 2013, p. 1702.
- ↑ Androgens in women
- ↑ Hansen, KR; Knowlton, NS; Thyer, AC; Charleston, JS; Soules, MR; Klein, NA (2008). "A new model of reproductive aging: the decline in ovarian non-growing follicle number from birth to menopause". Hum Reprod. 23 (3): 699–708. doi:10.1093/humrep/dem408. PMID 18192670.
- ↑ Rzepka-Górska, I; Tarnowski, B; Chudecka-Głaz, A; Górski, B; Zielińska, D; Tołoczko-Grabarek, A (2006). "Premature menopause in patients with BRCA1 gene mutation". Breast Cancer Res Treat. 100 (1): 59–63. doi:10.1007/s10549-006-9220-1. PMID 16773440.
- 1 2 Titus, S; Li, F; Stobezki, R; Akula, K; Unsal, E; Jeong, K; Dickler, M; Robson, M; Moy, F; Goswami, S; Oktay, K (2013). "Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans". Sci Transl Med. 5 (172): 172ra21. doi:10.1126/scitranslmed.3004925. PMC 5130338. PMID 23408054.
- ↑ Harris Bernstein, Carol Bernstein and Richard E. Michod (2011). Meiosis as an Evolutionary Adaptation for DNA Repair. Chapter 19 in DNA Repair. Inna Kruman editor. InTech Open Publisher. DOI: 10.5772/25117 http://www.intechopen.com/books/dna-repair/meiosis-as-an-evolutionary-adaptation-for-dna-repair
- ↑ "Ovarian Germ Cell Tumors Treatment". National Cancer Institute. Retrieved 2017-12-01.
- ↑ Seiden, Michael (2015). "Gynecologic Malignancies, Chapter 117". MGraw-Hill Medical. Archived from the original on September 10, 2017. Retrieved June 24, 2017.
- ↑ "Defining Cancer". National Cancer Institute. Archived from the original on 25 June 2014. Retrieved 10 June 2014.
- ↑ "NCI Dictionary of Cancer Terms". National Cancer Institute. Retrieved 2017-12-01.
- ↑ "MeSH Browser". meshb.nlm.nih.gov. Retrieved 2017-12-01.
- ↑ https://www.cancer.org/cancer/ovarian-cancer/treating/germ-cell-tumors.html
- ↑ Isachenko V, Lapidus I, Isachenko E, et al. (2009). "Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation". Reproduction. 138 (2): 319–27. doi:10.1530/REP-09-0039. PMID 19439559.
- 1 2 3 Oktay K, Oktem O (November 2008). "Ovarian cryopreservation and transplantation for fertility preservation for medical indications: report of an ongoing experience". Fertil. Steril. 93 (3): 762–8. doi:10.1016/j.fertnstert.2008.10.006. PMID 19013568.
- ↑ Livebirth after orthotopic transplantation of cryopreserved ovarian tissue The Lancet, Sep 24, 2004
- ↑ Lan C, Xiao W, Xiao-Hui D, Chun-Yan H, Hong-Ling Y (December 2008). "Tissue culture before transplantation of frozen-thawed human fetal ovarian tissue into immunodeficient mice". Fertil. Steril. 93 (3): 913–9. doi:10.1016/j.fertnstert.2008.10.020. PMID 19108826.
- 1 2 3 4 Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 383–385. ISBN 0-03-910284-X.
- ↑ https://www.britannica.com/science/animal-reproductive-system/Accessory-glands
- ↑ Moravec, František; Justine, Jean-Lou (2014). "Philometrids (Nematoda: Philometridae) in carangid and serranid fishes off New Caledonia, including three new species". Parasite. 21: 21. doi:10.1051/parasite/2014022. ISSN 1776-1042. PMC 4023622. PMID 24836940.

- ↑ Fitzpatrick, F. L. (1934). "Unilateral and bilateral ovaries in raptorial birds". The Wilson Bulletin. 46 (1): 19–22.
- ↑ Kinsky, F. C. (1971). "The consistent presence of paired ovaries in the Kiwi(Apteryx) with some discussion of this condition in other birds". Journal of Ornithology. 112 (3): 334–357. doi:10.1007/bf01640692.
Bibliography
- Venes, Donald (2013). Taber's cyclopedic medical dictionary. Philadelphia: F.A. Davis. ISBN 9780803629790.
External links
| Look up ovary in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Ovary. |