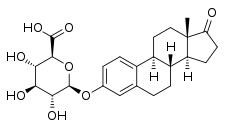
Estrone glucuronide
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3S,4S,5R,6S)-3,4,5-Trihydroxy-6-[[(8R,9S,13S,14S)-13-methyl-17-oxo-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-3-yl]oxy]oxane-2-carboxylic acid | |
| Other names
Estrone 3-glucuronide; Estrone 3-D-glucuronide; Estra-1,3,5(10)-triene-3-ol-17-one 3-D-glucuronoside | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C24H30O8 | |
| Molar mass | 446.50 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
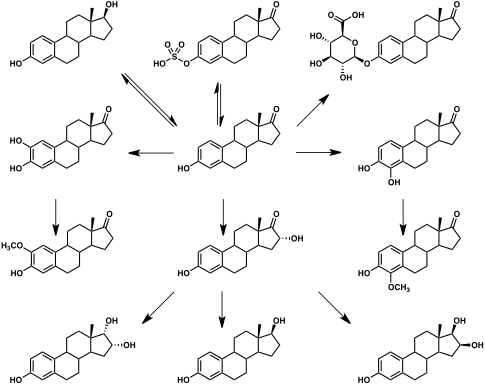
Estrone glucuronide, or estrone-3-D-glucuronide, is a conjugated metabolite of estrone.[1] It is formed from estrone in the liver by UDP-glucuronyltransferase via attachment of glucuronic acid and is eventually excreted in the urine by the kidneys.[1] It has much higher water solubility than does estrone.[1] Glucuronides are the most abundant estrogen conjugates and estrone glucuronide is the dominant metabolite of estradiol.[1]
When exogenous estradiol is administered orally, it is subject to extensive first-pass metabolism (95%) in the intestines and liver.[2][3] A single administered dose of estradiol is absorbed 15% as estrone, 25% as estrone sulfate, 25% as estradiol glucuronide, and 25% as estrone glucuronide.[2] Formation of estrogen glucuronide conjugates is particularly important with oral estradiol as the percentage of estrogen glucuronide conjugates in circulation is much higher with oral ingestion than with parenteral estradiol.[2] Estrone glucuronide can be reconverted back into estradiol, and a large circulating pool of estrogen glucuronide and sulfate conjugates serves as a long-lasting reservoir of estradiol that effectively extends its terminal half-life of oral estradiol.[2][3] In demonstration of the importance of first-pass metabolism and the estrogen conjugate reservoir in the pharmacokinetics of estradiol,[2] the terminal half-life of oral estradiol is 13 to 20 hours[4] whereas with intravenous injection its terminal half-life is only about 1 to 2 hours.[5]
See also
References
- 1 2 3 4 http://www.hmdb.ca/metabolites/HMDB04483
- 1 2 3 4 5 Oettel M, Schillinger E (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 268–. ISBN 978-3-642-60107-1.
- 1 2 Lauritzen C, Studd JW (22 June 2005). Current Management of the Menopause. CRC Press. pp. 364–. ISBN 978-0-203-48612-2.
- ↑ Stanczyk FZ, Archer DF, Bhavnani BR (June 2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–27. doi:10.1016/j.contraception.2012.12.011. PMID 23375353.
- ↑ Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. PMID 7169965.
- ↑ Buchsbaum HJ, ed. (2012). The Menopause (Clinical Perspectives in Obstetrics and Gynecology). New York, NY: Springer Science & Business Media. p. 64. ISBN 9781461255253.
- ↑ Kuhl H (August 2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric : the Journal of the International Menopause Society. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ↑ "EC 2.4.1.17 – glucuronosyltransferase and Organism(s) Homo sapiens". BRENDA. Technische Universität Braunschweig. January 2018. Retrieved 10 August 2018.