Testosterone enanthate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Delatestryl, others |
| Synonyms | TE; Testosterone heptanoate; Testosterone 17β-heptanoate; NSC-17591 |
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
Oral: very low Intramuscular: high |
| Metabolism | Liver |
| Elimination half-life | Intramuscular: 4–5 days[1] |
| Excretion | Urine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H40O3 |
| Molar mass | 400.603 g/mol |
| 3D model (JSmol) | |
| |
| |
Testosterone enanthate, sold under the brand name Delatestryl among others, is an androgen and anabolic steroid (AAS) medication which is used mainly in the treatment of low testosterone levels in men.[2][3][4] It is also used in hormone therapy for transgender men.[5] It is given by injection into muscle usually once every two to four weeks.[4][6][1]
Side effects of testosterone enanthate include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[4] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[7][4] It has strong androgenic effects and moderate anabolic effects, which make it useful for producing masculinization and suitable for androgen replacement therapy.[4] Testosterone enanthate is a testosterone ester and a long-lasting prodrug of testosterone in the body.[6][2][3] Because of this, it is considered to be a natural and bioidentical form of testosterone.[8]
Testosterone enanthate was introduced for medical use in 1954.[9][3] Along with testosterone cypionate, testosterone undecanoate, and testosterone propionate, it is one of the most widely used testosterone esters.[7][3][4] In addition to its medical use, testosterone enanthate is used to improve physique and performance.[4] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[4]
Medical uses
Testosterone enanthate is used primarily in androgen replacement therapy.[3] It is the most widely used form of testosterone in androgen replacement therapy.[3] The medication is specifically approved, in the United States, for the treatment of hypogonadism in men, delayed puberty in boys, and breast cancer in women.[10] It is also used in masculinizing hormone therapy for transgender men.[5]
Side effects
Side effects of testosterone enanthate include virilization among others.[4]
Pharmacology
Pharmacodynamics
Testosterone enanthate is a prodrug of testosterone and is an androgen and anabolic–androgenic steroid (AAS). That is, it is an agonist of the androgen receptor (AR).
Pharmacokinetics
Testosterone enanthate has an elimination half-life of 4.5 days and a mean residence time of 8.5 days when used as a depot intramuscular injection.[1] It requires frequent administration of approximately once per week, and large fluctuations in testosterone levels result with it, with levels initially being elevated and supraphysiological.[1]
Chemistry
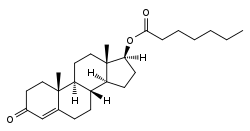
Testosterone enanthate, or testosterone 17β-heptanoate, is a synthetic androstane steroid and a derivative of testosterone.[11][12] It is an androgen ester; specifically, it is the C17β enanthate (heptanoate) ester of testosterone.[11][12]
History
Testosterone enanthate was first introduced for medical use in the United States in 1954 under the brand name Delatestryl.[9][3]
Society and culture
Generic names
Testosterone enanthate is the generic name of the drug and its USAN and BAN.[11][12][13][14] It has also referred to as testosterone heptanoate.[11][12][13][14]
Brand names
Testosterone enanthate is marketed primarily under the brand name Delatestryl.[11][12][13][14] It is or has been marketed under a variety of other brand names as well including Andro LA, Andropository, Depandro, Durathate, Everone, Testrin, Testostroval, and Testro LA among others.[11][12][13][14]
Availability
Testosterone enanthate is available in the United States and widely elsewhere throughout the world.[15][12][14]
Legal status
Testosterone enanthate, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act and a schedule IV controlled substance in Canada under the Controlled Drugs and Substances Act.[16][17]
Research
As of October 2017, an auto-injection formulation of testosterone enanthate is in preregistration for the treatment of hypogonadism in the United States.[18]
References
- 1 2 3 4 Anita H. Payne; Matthew P. Hardy (28 October 2007). The Leydig Cell in Health and Disease. Springer Science & Business Media. pp. 423–. ISBN 978-1-59745-453-7.
- 1 2 Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (26 July 2012). Testosterone: Action, Deficiency, Substitution. Cambridge University Press. pp. 315–. ISBN 978-1-107-01290-5.
- 1 2 3 4 5 6 7 Eberhard Nieschlag; Hermann M. Behre; Susan Nieschlag (13 January 2010). Andrology: Male Reproductive Health and Dysfunction. Springer Science & Business Media. pp. 442–. ISBN 978-3-540-78355-8.
- 1 2 3 4 5 6 7 8 9 William Llewellyn (2011). Anabolics. Molecular Nutrition Llc. pp. 208–211. ISBN 978-0-9828280-1-4.
- 1 2 Irwig MS (2017). "Testosterone therapy for transgender men". Lancet Diabetes Endocrinol. 5 (4): 301–311. doi:10.1016/S2213-8587(16)00036-X. PMID 27084565.
- 1 2 Kenneth L. Becker (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. pp. 1185, 1187. ISBN 978-0-7817-1750-2.
- 1 2 Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ↑ Santoro N, Braunstein GD, Butts CL, Martin KA, McDermott M, Pinkerton JV (2016). "Compounded Bioidentical Hormones in Endocrinology Practice: An Endocrine Society Scientific Statement". J. Clin. Endocrinol. Metab. 101 (4): 1318–43. doi:10.1210/jc.2016-1271. PMID 27032319.
- 1 2 William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 3171–. ISBN 978-0-8155-1856-3.
- ↑ https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/009165s031lbl.pdf
- 1 2 3 4 5 6 J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 641–642. ISBN 978-1-4757-2085-3.
- 1 2 3 4 5 6 7 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 1002–1004. ISBN 978-3-88763-075-1.
- 1 2 3 4 I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. ISBN 978-94-011-4439-1.
- 1 2 3 4 5 https://www.drugs.com/international/testosterone.html
- ↑ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 17 December 2016.
- ↑ Steven B. Karch, MD, FFFLM (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- ↑ Linda Lane Lilley; Julie S. Snyder; Shelly Rainforth Collins (5 August 2016). Pharmacology for Canadian Health Care Practice. Elsevier Health Sciences. pp. 50–. ISBN 978-1-77172-066-3.
- ↑ http://adisinsight.springer.com/drugs/800037740
External links