Hydroxyzine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /haɪˈdrɒksɪziːn/ |
| Trade names | Atarax, Vistaril, others |
| Synonyms | UCB-4492 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682866 |
| Pregnancy category | |
| Routes of administration | By mouth, intramuscular injection |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | High |
| Protein binding | 93% |
| Metabolism | Hepatic |
| Metabolites | Cetirizine, others |
| Elimination half-life |
Adults: 20.0 hours[1][2] Children: 7.1 hours[1] |
| Excretion | Urine, feces |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.000.630 |
| Chemical and physical data | |
| Formula | C21H27ClN2O2 |
| Molar mass | 374.904 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Hydroxyzine, sold under the brand names Atarax and Vistaril among others, is a first-generation antihistamine. It was first synthesized by Union Chimique Belge in 1956 and was marketed by Pfizer in the United States later the same year[3] and is still widely used today.
Because of its antihistamine effects, it can be used for the treatment of severe cases of itching, hyperalgesia, and motion sickness-induced nausea; it has also been used in some cases to relieve the effects of opioid withdrawal.[4][5] Even though it is an effective sedative, hypnotic, and anxiolytic, it shares virtually none of the abuse, dependence, addiction, and toxicity potential of other drugs used for the same range of therapeutic reasons. Hydroxyzine has also been used to potentiate the analgesia of opioids and to alleviate some of their side effects, such as itching, nausea, and vomiting.
Due to its antagonistic effects on several receptor systems in the brain, hydroxyzine also has anxiolytic, antiobsessive, and antipsychotic activity.[6] Today it is used primarily for the symptomatic relief of anxiety and tension associated with psychoneurosis and as an adjunct in organic disease states in which anxiety is manifested.
Other drugs related to hydroxyzine are cyclizine, buclizine, and meclizine, and they share all or most of the benefits, indications, contraindications, cautions, and side effects of hydroxyzine. The second-generation antihistamine cetirizine is in fact one of the metabolites of hydroxyzine produced in the human body. Unlike hydroxyzine, cetirizine is not reported to appreciably cross the blood-brain barrier, but it has been reported to be associated with dystonic reactions as well as sedation. Therefore, it has a narrower spectrum of effects, making it an effective antihistamine but removing some or all of the anxiolytic and other psychoactive properties, but it may cause dystonic reactions and drowsiness in some patients.
Medical uses
Hydroxyzine is classified as an antihistamine and anxiolytic and is also used as a tranquilizer, especially common in dentistry and less so in medicine, where for many years it has been preferred in combination with opioids due to its ability to counteract the side effects of opioid pain medications, namely itchiness and nausea, and the fact that its sedative properties complement the pain-relieving effects of opioids.
Hydroxyzine is prescribed when the onset of an organic disease state manifests through anxiety, as generalized anxiety disorder, or in other more serious cases as psychoneurosis, and is therefore prescribed as a means of regulating normal function. Hydroxyzine has shown to be as effective as the benzodiazepine drug bromazepam in the treatment of generalised anxiety disorder.[7] A systematic review concluded that compared with other anxiolytic agents (benzodiazepines and buspirone), hydroxyzine was equivalent in efficacy, acceptability, and tolerability.[8]
Hydroxyzine can also be used for the treatment of allergic conditions, such as chronic urticaria, atopic or contact dermatoses, and histamine-mediated pruritus. These have also been confirmed in both recent and past studies to have no adverse effects on the liver, blood, nervous system, or urinary tract.[9]
Use of hydroxyzine for premedication as a sedative has no effects on tropane alkaloids, such as atropine, but may, following general anesthesia, potentiate meperidine and barbiturates, and use in pre-anesthetic adjunctive therapy should be modified depending upon the state of the individual.[9]
In other cases, the usage of hydroxyzine is as a form of non-barbiturate tranquilizer[10] used in the pre-operative sedation and treatment of neurological disorders, such as psychoneurosis and other forms of anxiety or tension states.[10]
For dentistry and obstetrics as well as other surgeries and procedures and acute pain situations like accidents, hydroxyzine is useful as a first-line anxiolytic and opioid adjunct because it lacks both antagonism and synergy with benzodiazepines and scopolamine, allowing either of these agents to be used simultaneously or later in the procedure if need be.
Contraindications
The administration of hydroxyzine in large amounts by ingestion or intramuscular administration during the onset of pregnancy can cause fetal abnormalities—when administered to pregnant rats, mice and rabbits, hydroxyzine caused abnormalities such as hypogonadism with doses significantly above that of the human therapeutic range.[11] In humans, a significant dose has not yet been established in studies, and by default, the FDA has introduced contraindication guidelines in regard to hydroxyzine.[11] Similarly the use in those at risk from or showing previous signs of hypersensitivity is also contraindicated.[11] Hydroxyzine is contraindicated for intravenous (IV) injection, as it has shown to cause hemolysis.
Other contraindications include the administration of hydroxyzine alongside depressants and other compounds which affect the central nervous system.[11] and if absolutely necessary, it should only be administered concomitantly in small doses.[11] If administered in small doses with other substances, such as mentioned, then patients should refrain from using dangerous machinery, motor vehicles or any other practice requiring absolute concentration, in accordance with safety law.[11]
Studies have also been conducted which show that long-term prescription of hydroxyzine can lead to tardive dyskinesia after years of use, but effects related to dyskinesia have also anecdotally been reported after periods of 7.5 months,[12] such as continual head rolling, lip licking and other forms of athetoid movement. In certain cases, elderly patients' previous interactions with phenothiazine derivatives or pre-existing neuroleptic treatment may have had some contribution towards dyskinesia at the administration of hydroxyzine due to hypersensitivity caused due to the prolonged treatment,[12] and therefore some contraindication is given to the short-term administration of hydroxyzine to those with previous phenothiazine use.[12]
Side effects
Several reactions have been noted in manufacturer guidelines — deep sleep, incoordination, sedation, calmness, and dizziness have been reported in children and adults, as well as others such as hypotension, tinnitus, and headaches.[13] Gastro-intestinal effects have also been observed, as well as less serious effects such as dryness of the mouth and constipation caused by the mild antimuscarinic properties of hydroxyzine.[13]
Central nervous system problems such as hallucinations or confusion have been observed in rare cases, attributed mostly to overdosage.[14][13] Such properties have been attributed to hydroxyzine in several cases, particularly in patients treated for neuropsychological disorders, as well as in cases where overdoses have been observed. While there are reports of the "hallucinogenic" or "hypnotic" properties of hydroxyzine, several clinical data trials have not reported such side effects from the sole consumption of hydroxyzine, but rather, have described its overall calming effect described through the stimulation of areas within the formatio reticularis. The hallucinogenic or hypnotic properties have been described as being an additional effect from overall central nervous system suppression by other CNS agents, such as lithium or ethanol.[15]
The effect of hydroxyzine has also been tested on the ability of humans in the registration and storage of memory, and was used in comparison with relatively safe drugs, such as lorazepam, to illustrate the effects of benzodiazepines, which are thought to have adverse effects on the capacity of memory storage. Hydroxyzine was found to have no adverse effects on memory in relation to lorazepam, which caused several deficiencies in the capacity of memory storage.[16]
In a comparative study with lorazepam on memory effects, patients who had taken hydroxyzine experienced sedative effects like drowsiness, but recalled that they felt capable, attentive and able to continue with a memory test under these conditions.[17] Conversely, those under the effects of lorazepam felt unable to continue due to the fact they felt out of control with its effects; 8 out of 10 patients describing tendencies of problems with balance and control of simple motor functions.[17]
Somnolence with or without vivid dreams or nightmares may occur in users with antihistamine sensitivities in combination with other CNS depressants. Hydroxyzine exhibits anxiolytic and sedative properties in many psychiatric patients. Other studies have suggested that hydroxyzine acts as an acute hypnotic, reducing sleep onset latency and increasing sleep duration — also showing that some drowsiness did occur. This was observed more in female patients, who also had greater hypnotic response.[18]
Because of potential for more severe side effects, this drug is on the list to avoid in the elderly.[19]
In 2015, the European Medicines Agency (EMA) announced a small but definite risk of QT prolongation associated with the use of hydroxyzine. This side effect is more likely to occur in people with pre-existing cardiac disease, or with the use of other medicines known to prolong the QT interval.
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| 5-HT2A | 170 (IC50) | Rat | [21] |
| 5-HT2C | ND | ND | ND |
| α1 | 460 (IC50) | Rat | [21] |
| D1 | >10,000 | Mouse | [22] |
| D2 | 378 560 (IC50) | Mouse Rat | [22] [21] |
| H1 | 2.0–19 6.4 100 (IC50) | Human Bovine Rat | [23][24][25] [26] [21] |
| H2 | ND | ND | ND |
| H3 | ND | ND | ND |
| H4 | >10,000 | Human | [24] |
| mACh | 4,600 10,000 10,000 (IC50) 6,310 (pA2) 3,800 | Human Mouse Rat Guinea pig Bovine | [27] [22] [21] [28] [26] |
| VDCC | ≥3,400 (IC50) | Rat | [21] |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | |||
Hydroxyzine's predominant mechanism of action is as a potent and selective histamine H1 receptor inverse agonist.[29][30] This action is responsible for its antihistamine and sedative effects.[29][30] Unlike many other first-generation antihistamines, hydroxyzine has very low affinity for the muscarinic acetylcholine receptors, and in accordance, has low or no propensity for producing anticholinergic side effects.[26][30][31][32] In addition to its antihistamine activity, hydroxyzine has also been shown to act more weakly as an antagonist of the serotonin 5-HT2A receptor, the dopamine D2 receptor, and the α1-adrenergic receptor.[21][29] The weak antiserotonergic effects of hydroxyzine likely underlie its usefulness as an anxiolytic,[33] as other antihistamines without such properties have not been found to be effective in the treatment of anxiety.[34]
Hydroxyzine crosses the blood–brain barrier easily and exerts effects in the central nervous system.[29] A positron emission tomography (PET) study found that brain occupancy of the H1 receptor was 67.6% for a single 30 mg dose of hydroxyzine.[35] In addition, subjective sleepiness correlated well with the brain H1 receptor occupancy.[35] PET studies with antihistamines have found that brain H1 receptor occupancy of more than 50% is associated with a high prevalence of somnolence and cognitive decline, whereas brain H1 receptor occupancy of less than 20% is considered to be non-sedative.[36]
Pharmacokinetics
Hydroxyzine can be administered orally or via intramuscular injection. When given orally, hydroxyzine is rapidly absorbed from the gastrointestinal tract. The effect of hydroxyzine is notable in 30 minutes.
Pharmacokinetically, hydroxyzine is rapidly absorbed and distributed in oral and intramuscular administration, and is metabolized in the liver; the main metabolite (45%), cetirizine, is formed through oxidation of the alcohol moiety to a carboxylic acid by alcohol dehydrogenase, and overall effects are observed within one hour of administration. Higher concentrations are found in the skin than in the plasma. Cetirizine, although less sedating, is non-dialyzable and possesses similar anti-histaminergic properties. The other metabolites identified include a N-dealkylated metabolite, and an O-dealkylated 1/16 metabolite with a plasma half-life of 59 hours. These pathways are mediated principally by CYP3A4 and CYP3A5.[37] "In animals, hydroxyzine and its metabolites are excreted in feces via biliary elimination."[38]
Administration in geriatrics differs from the administration of hydroxyzine in younger patients; according to the FDA, there have not been significant studies made (2004), which include population groups over 65, which provide a distinction between elderly aged patients and other younger groups. Hydroxyzine should be administered carefully in the elderly with consideration given to possible reduced elimination.[14]
Similarly, the use of sedating drugs alongside hydroxyzine can cause oversedation and confusion if administered in large amounts—any form of treatment alongside sedatives should be done under supervision of a doctor.[10][14]
The Tmax of hydroxyzine is about 2.0 hours in both adults and children and its elimination half-life is around 20.0 hours in adults and 7.1 hours in children.[1][2] Another study found that the elimination half-life of hydroxyzine in adults was as short as 3 hours, but this may have simply been due to methodological limitations.[39]
Chemistry
Hydroxyzine is a member of the diphenylmethylpiperazine class of antihistamines.
Analogues
Analogues of hydroxyzine include buclizine, cetirizine, cinnarizine, cyclizine, etodroxizine, meclizine, and pipoxizine among others.
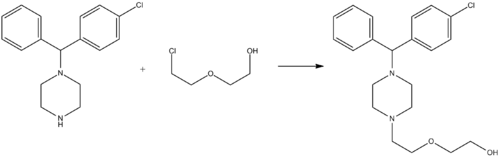
Synthesis
Hydroxyzine is synthesized by the alkylation of 1-(4-chlorobenzohydril)piperazine with 2-(2-hydroxyethoxy)ethylchloride:[40]
Brand names
Hydroxyzine preparations require a doctor's prescription. The drug is available in two formulations, the pamoate and the dihydrochloride or hydrochloride salts. Vistaril, Equipose, Masmoran, and Paxistil are preparations of the pamoate salt, while Atarax, Alamon, Aterax, Durrax, Tran-Q, Orgatrax, Quiess, and Tranquizine are of the hydrochloride salt.
References
- 1 2 3 Paton DM, Webster DR (1985). "Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines)". Clin Pharmacokinet. 10 (6): 477–97. doi:10.2165/00003088-198510060-00002. PMID 2866055.
- 1 2 Simons FE, Simons KJ, Frith EM (January 1984). "The pharmacokinetics and antihistaminic of the H1 receptor antagonist hydroxyzine". The Journal of Allergy and Clinical Immunology. 73 (1 Pt 1): 69–75. doi:10.1016/0091-6749(84)90486-x. PMID 6141198.
- ↑ Shorter, Edward (2009). Before Prozac: the troubled history of mood disorders in psychiatry. Oxford [Oxfordshire]: Oxford University Press. ISBN 0-19-536874-6.
- ↑ FDA DrugInfo: http://dailymed.nlm.nih.gov/dailymed/archives/fdaDrugInfo.cfm?archiveid=51459
- ↑ "Hydroxyzine Facts and Comparisons at Drugs.com".
- ↑ SCHRAM, WS (March 1959). "Use of hydroxyzine in psychosis". Diseases of the Nervous System. 20 (3): 126–9. PMID 13639831.
- ↑ Llorca PM, Spadone C, Sol O, et al. (November 2002). "Efficacy and safety of hydroxyzine in the treatment of generalized anxiety disorder: a 3-month double-blind study" (PDF). J Clin Psychiatry. 63 (11): 1020–7. doi:10.4088/JCP.v63n1112. PMID 12444816.
- ↑ Giuseppe Guaiana1, Corrado Barbui, Andrea Cipriani (8 December 2010). "Hydroxyzine for generalised anxiety disorder". The Cochrane Library. Cochrane Depression, Anxiety and Neurosis Group. doi:10.1002/14651858.CD006815.pub2.
- 1 2 United States Food & Drug Administration, (2004), p1
- 1 2 3 Dolan, C. M., (1958)
- 1 2 3 4 5 6 United States Food & Drug Administration, (2004), p2
- 1 2 3 Clark, B. G., Araki, M., et al. (1976)
- 1 2 3 UCB South-Africa, et al., (2004)
- 1 2 3 United States Food & Drug Administration, (2004), p3
- ↑ Anderson, P. O., Knoben, J. E., et al. (2002), p794-796
- ↑ Brabander, A. DE, Debert, W., (1990), p1
- 1 2 Brabander, A. DE, Debert, W., (1990), p3
- ↑ Alford, C.; N. Rombautt, J. Jones, S. Foley, C. Idzikowskit and I. Hindmarch (1992).
- ↑ NCQA’s HEDIS Measure: Use of High Risk Medications in the Elderly, "Archived copy" (PDF). Archived from the original (PDF) on 2010-02-01. Retrieved 2010-02-22.
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- 1 2 3 4 5 6 7 Snowman AM, Snyder SH (December 1990). "Cetirizine: actions on neurotransmitter receptors". The Journal of Allergy and Clinical Immunology. 86 (6 Pt 2): 1025–8. doi:10.1016/S0091-6749(05)80248-9. PMID 1979798.
- 1 2 3 Haraguchi K, Ito K, Kotaki H, Sawada Y, Iga T (June 1997). "Prediction of drug-induced catalepsy based on dopamine D1, D2, and muscarinic acetylcholine receptor occupancies". Drug Metabolism and Disposition. 25 (6): 675–84. PMID 9193868.
- ↑ Gillard M, Van Der Perren C, Moguilevsky N, Massingham R, Chatelain P (February 2002). "Binding characteristics of cetirizine and levocetirizine to human H(1) histamine receptors: contribution of Lys(191) and Thr(194)". Molecular Pharmacology. 61 (2): 391–9. doi:10.1124/mol.61.2.391. PMID 11809864.
- 1 2 Lim HD, van Rijn RM, Ling P, Bakker RA, Thurmond RL, Leurs R (2005). "Evaluation of histamine H1-, H2-, and H3-receptor ligands at the human histamine H4 receptor: identification of 4-methylhistamine as the first potent and selective H4 receptor agonist". J. Pharmacol. Exp. Ther. 314 (3): 1310–21. doi:10.1124/jpet.105.087965. PMID 15947036.
- ↑ Anthes JC, Gilchrest H, Richard C, Eckel S, Hesk D, West RE, Williams SM, Greenfeder S, Billah M, Kreutner W, Egan RE (2002). "Biochemical characterization of desloratadine, a potent antagonist of the human histamine H(1) receptor". Eur. J. Pharmacol. 449 (3): 229–37. doi:10.1016/s0014-2999(02)02049-6. PMID 12167464.
- 1 2 3 Kubo N, Shirakawa O, Kuno T, Tanaka C (March 1987). "Antimuscarinic effects of antihistamines: quantitative evaluation by receptor-binding assay". Japanese Journal of Pharmacology. 43 (3): 277–82. doi:10.1254/jjp.43.277. PMID 2884340.
- ↑ Cusack B, Nelson A, Richelson E (1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology. 114 (4): 559–65. doi:10.1007/bf02244985. PMID 7855217.
- ↑ Orzechowski RF, Currie DS, Valancius CA (January 2005). "Comparative anticholinergic activities of 10 histamine H1 receptor antagonists in two functional models". European Journal of Pharmacology. 506 (3): 257–64. doi:10.1016/j.ejphar.2004.11.006. PMID 15627436.
- 1 2 3 4 J. Szepietowski; E. Weisshaar (30 August 2016). Itch - Management in Clinical Practice. Karger Medical and Scientific Publishers. pp. 1–. ISBN 978-3-318-05889-5.
- 1 2 3 Hosák, Ladislav; Hrdlička, Michal (1 February 2017). Psychiatry and Pedopsychiatry. Charles University in Prague, Karolinum Press. pp. 364–. ISBN 978-80-246-3378-7.
- ↑ Berger, F. M. (1957). "THE CHEMISTRY AND MODE OF ACTION OF TRANQUILIZING DRUGS". Annals of the New York Academy of Sciences. 67 (10): 685–700. doi:10.1111/j.1749-6632.1957.tb46006.x. ISSN 0077-8923.
- ↑ K. D. Tripathi (2013). Essentials of Medical Pharmacology. JP Medical Ltd. p. 165. ISBN 978-93-5025-937-5.
- ↑ Barbara Olasov Rothbaum; Stein, Dan J.; Hollander, Eric (2009). Textbook of Anxiety Disorders. American Psychiatric Publishing, Inc. ISBN 1-58562-254-0.
- ↑ Lamberty Y, Gower AJ (September 2004). "Hydroxyzine prevents isolation-induced vocalization in guinea pig pups: comparison with chlorpheniramine and immepip". Pharmacology Biochemistry and Behavior. 79 (1): 119–24. doi:10.1016/j.pbb.2004.06.015. PMID 15388291.
- 1 2 Tashiro M, Kato M, Miyake M, Watanuki S, Funaki Y, Ishikawa Y, Iwata R, Yanai K (2009). "Dose dependency of brain histamine H(1) receptor occupancy following oral administration of cetirizine hydrochloride measured using PET with [11C]doxepin". Hum Psychopharmacol. 24 (7): 540–8. doi:10.1002/hup.1051. PMID 19697300.
- ↑ Yanai K, Tashiro M (2007). "The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies". Pharmacol. Ther. 113 (1): 1–15. doi:10.1016/j.pharmthera.2006.06.008. PMID 16890992.
- ↑ "Ucerax (hydroxyzine hydrochloride) 25 mg film-coated tablets. Summary of product characteristics" (PDF). Irish Medicines Board. Retrieved 9 February 2014.
- ↑ "The extent of renal excretion of VISTARIL has not been determined""Archived copy" (PDF). Archived from the original (PDF) on 2007-07-03. Retrieved 2007-07-03.
- ↑ Sam Kacew (30 November 1989). Drug Toxicity & Metabolism In Pediatrics. CRC Press. pp. 257–. ISBN 978-0-8493-4564-7.
- ↑ H. Morren, U.S. Patent 2,899,436 (1959); H. Morren, DE 1049383 (1954); H. Morren, DE 1061786 (1954); H. Morren, DE 1068262 (1954); H. Morren, DE 1072624 (1954); H. Morren, DE 1075116 (1954).
External links
Publications
- Hutcheon, D. E.; D.L. Morris; A. Scriabine (December 1956). "Cardiovascular action of hydroxyzine (Atarax)". J Pharmacol Exp Ther. 118 (4): 451–460. PMID 13385806.
- Dolan, C. M. (June 1958). "MANAGEMENT OF EMOTIONAL DISTURBANCES—Use of Hydroxyzine (Atarax®) in General Practice". Calif Med. 88 (6): 443–444. PMC 1512309. PMID 13536863.
- Pfizer Labs, Division of Pfizer Inc, NY, NY 10017 (2004). Vistaril (hydroxyzine pamoate) Capsules and Oral Suspension (pdf). United States Food and Drug Administration. Retrieved 2007-03-09.
- Anderson, Philip O.; James E. Knoben; William G. Troutman (2002). Handbook of Clinical Drug Data. McGraw-Hill Medical. ISBN 0-07-136362-9.
- de Brabander, A.; W. Deberdt (1990). "Effect of Hydroxyzine on Attention and Memory". Human Psychopharmacology. John Wiley & Sons. 5 (4): 357–362. doi:10.1002/hup.470050408. Retrieved 2007-03-09.
- Clark, B. G.; M. Araki; H. W. Brown (1982). "Hydroxyzine-Associated Tardive Dyskinesia". Ann. Neurol. 11 (4): 435. doi:10.1002/ana.410110423. PMID 7103423.
- Porsolt, R. D.; P. Martin; A. Lenegre; S. Frornage; and C.E. Giurgea (1989). "Prevention of "Learned Helplessness" in the Rat by Hydroxyzine". Drug Dev. Res. 17 (3): 227–236. doi:10.1002/ddr.430170306. Retrieved 2007-03-10.
- Alford, C.; N. Rombautt; J. Jones; S. Foley; C. Idzikowskit; I. Hindmarch (1992). "Acute Effects of Hydroxyzine on Nocturnal Sleep and Sleep Tendency the Following Day: a C-EEG Study". Human Psychopharmacology. 7 (1): 25–35. doi:10.1002/hup.470070104. Retrieved 2007-03-10.
Web pages
- RxList; et al. (2004). "Atarax Indications, Dosage, Storage, Stability". RxList - The internet drug index. Retrieved 2007-03-09.
- Medscape (2004). "Vistaril Oral: Monograph - Hydroxyzine Hydrochloride, Hydroxyzine Pamoate". medscape.com. Retrieved 2007-03-09.
- pfizer (2004). "Non-print version of vistaril fact sheet" (PDF). Archived from the original (PDF) on 2007-07-03. Retrieved 2007-07-03.
- U.S. National Library of Medicine: Drug Information Portal - Hydroxyzine