Lorcaserin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Belviq |
| Synonyms | APD-356 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a613014 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 70%[1] |
| Metabolism | Hepatic (extensive)[1] |
| Elimination half-life | 11 hours[1] |
| Excretion | Renal (92.3%), Faecal (2.2%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard |
100.237.138 |
| Chemical and physical data | |
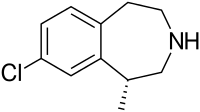
| Formula | C11H14ClN |
| Molar mass | 195.688 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Lorcaserin, currently marketed under the trade name Belviq[2][3] and previously Lorqess during development,[4][5] is a weight-loss drug developed by Arena Pharmaceuticals. It reduces appetite by activating a type of serotonin receptor known as the 5-HT2C receptor in a region of the brain called the hypothalamus, which is known to control appetite.[6]
Medical uses
Lorcaserin is used long term for weight loss in those who are obese.[7]
Side effects
In December 2012, the US Drug Enforcement Administration proposed classifying lorcaserin as a Schedule IV drug because it has hallucinogenic properties at higher than approved doses and users could develop psychiatric dependencies on the drug.[8][9] On 7 May 2013, the US Drug Enforcement Administration classified lorcaserin as a Schedule IV drug[10] under the Controlled Substances Act.[8]
There has been concern that lorcaserin can cause cardiac valvulopathy based upon the reports of subjects taking the drug in Phase 2 trials. However, a Phase 3 clinical trial of the drug was conducted and the results published in the October 2014 Postgraduate Medicine journal, a peer-reviewed medical journal for physicians. These results found no statistically significant differences in valvulopathy rates compared to control, being 2.4% for the drug subjects and 2.0% for controls.[11]
Mechanism of action
Lorcaserin is a selective 5-HT2C receptor agonist,[12] and in vitro testing of the drug showed reasonable selectivity for 5-HT2C over other related targets.[13][14][15] 5-HT2C receptors are located almost exclusively in the brain, and can be found in the choroid plexus, cortex, hippocampus, cerebellum, amygdala, thalamus, and hypothalamus. The activation of 5-HT2C receptors in the hypothalamus is supposed to activate proopiomelanocortin (POMC) production and consequently promote weight loss through satiety.[16] This hypothesis is supported by clinical trials and other studies. While it is generally thought that 5-HT2C receptors help to regulate appetite as well as mood, and endocrine secretion,[17] the exact mechanism of appetite regulation is not yet known. Lorcaserin has shown 100x selectivity for 5-HT2C versus the closely related 5-HT2B receptor, and 17x selectivity over the 5-HT2A receptor.[18][19]
| Receptor[1] | EC50 [nM] | Ki[nM] |
|---|---|---|
| 5-HT2C | 39 | 13 |
| 5-HT2B | 2380 | 147 |
| 5-HT2A | 553 | 92 |
Approval history
On 22 December 2009 a New Drug Application (NDA) was submitted to the Food and Drug Administration (FDA) in the United States.[20] On 16 September 2010, an FDA advisory panel voted to recommend against approval of the drug based on concerns over both safety and efficacy.[21] In October 2010, the FDA stated that it could not approve the application for lorcaserin in its present form.[22]
Lorcaserin had a Prescription Drug User Fee Act (PDUFA) date of 22 October 2010.[23] On 16 September 2010, a federal advisory committee voted against recommending approval for lorcaserin. In their 9-5 vote, the committee raised concerns about the safety of the drug, particularly the findings of tumors in rats.[24] On 23 October 2010, the FDA decided not to approve the drug based on the available data. This was not only because cancer promoting properties could not be ruled out, but also because the weight loss efficacy was considered "marginal."[22]
After additional studies were completed and additional information submitted to the FDA, an advisory panel was convened on 10 May 2012. The advisory panel voted 19-4-1 to recommend lorcaserin to the FDA. The FDA stated that the weight loss data passed FDA standards for efficacy and that the drug did not have cancer risks based on clarifications in the data. The FDA panelist recommended that postmarketing studies regarding potential heart valve issues be completed. The FDA has not stated one way or the other whether they believe this is necessary at this time although no related safety markers have been indicated during clinical studies. On 27 June 2012, the FDA officially approved lorcaserin for use in the treatment of obesity for some adults.[25][26]
On 10 May 2012, after a new round of studies submitted by Arena, an FDA panel voted to recommend lorcaserin with certain restrictions and patient monitoring. The restrictions include patients with a BMI of over 30, or with a BMI over 27 and a comorbidity such as high blood pressure or type 2 diabetes.[27] On 27 June 2012, the FDA officially approved lorcaserin for use in the treatment of obesity for adults with a BMI equal to or greater than 30 or adults with a BMI of 27 or greater who "have at least one weight-related health condition, such as high blood pressure, type 2 diabetes, or high cholesterol."[2][25]
See also
References
- 1 2 3 4 5 "BELVIQ (lorcaserin hydrochloride) tablet [Eisai, Inc]". DailyMed. Eisai, Inc. August 2012. Archived from the original on 21 October 2013. Retrieved 21 October 2013.
- 1 2 "FDA approves Arena obesity drug; first in 13 years". Reuters. 27 June 2012. Archived from the original on 24 September 2015.
- ↑ "Belviq". Trademarkia. 23 Jun 2011. Archived from the original on 30 June 2012. Retrieved 27 June 2012.
- ↑ "Lorqess". EvaluatePharma. 7 September 2010. Retrieved 13 September 2010.
- ↑ "Lorqess". Trademarkia. 25 March 2010. Archived from the original on 6 September 2010. Retrieved 13 September 2010.
- ↑ Shukla, AP; Kumar, RB; Aronne, LJ (2015). "Lorcaserin Hcl for the treatment of obesity". Expert Opinion on Pharmacotherapy. 16 (16): 2531–8. doi:10.1517/14656566.2015.1096345. PMID 26472579.
- ↑ Bray, GA; Frühbeck, G; Ryan, DH; Wilding, JP (7 May 2016). "Management of obesity". Lancet. 387 (10031): 1947–56. doi:10.1016/S0140-6736(16)00271-3. PMID 26868660.
- 1 2 Wilson, Megan R. (December 19, 2012). "Reg Watch". The Hill. Archived from the original on March 20, 2013.
- ↑ "Schedules of Controlled Substances: Placement of Lorcaserin into Schedule IV".
- ↑ "DEPARTMENT OF JUSTICE Drug Enforcement Administration 21 CFR Part 1308, Placement of Lorcaserin into Schedule IV".
- ↑ "BELVIQ and BELVIQ XR HCP Home Page". www.belviqhcp.com. Archived from the original on 2016-06-16.
- ↑ Thomsen, W. J.; Grottick, A. J.; Menzaghi, F.; Reyes-Saldana, H.; Espitia, S.; Yuskin, D.; Whelan, K.; Martin, M.; Morgan, M.; Chen, W.; Al-Shamma, H.; Smith, B.; Chalmers, D.; Behan, D. (2008). "Lorcaserin, a Novel Selective Human 5-Hydroxytryptamine2C Agonist: in Vitro and in Vivo Pharmacological Characterization". Journal of Pharmacology and Experimental Therapeutics. 325 (2): 577–587. doi:10.1124/jpet.107.133348. PMID 18252809.
- ↑ US patent 6953787, Brian Smith, Jeffrey Smith, "5HT2c receptor modulators", published 2003-10-04, issued 2005-11-10
- ↑ US patent 7704993, Brian Smith, Charles A. Gilson, III, Jeffrey Schultz, Jeffrey Smith, "Benzazepine derivatives and methods of prophylaxis or treatment of 5ht2c receptor associated diseases", published 2004-16-06, issued 2010-27-04
- ↑ US patent 8207158, Brian Smith, Jeffrey Smith, "5HT2c receptor modulators", published 2011-27-05, issued 2012-26-06
- ↑ Spreitzer, Helmut (13 September 2010). "Lorcaserin". Österreichische Apothekerzeitung (in German). 64 (19): 1083.
- ↑ Millan, M. J. (2005). "Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies". Thérapie. 60 (5): 441–460. doi:10.2515/therapie:2005065. PMID 16433010. Archived from the original on 2015-08-28.
- ↑ Smith, B. M.; Smith, J. M.; Tsai, J. H.; Schultz, J. A.; Gilson, C. A.; Estrada, S. A.; Chen, R. R.; Park, D. M.; Prieto, E. B.; Gallardo, C. S.; Sengupta, D.; Thomsen, W. J.; Saldana, H. R.; Whelan, K. T.; Menzaghi, F.; Webb, R. R.; Beeley, N. R. A. (2005). "Discovery and SAR of new benzazepines as potent and selective 5-HT2C receptor agonists for the treatment of obesity". Bioorganic & Medicinal Chemistry Letters. 15 (5): 1467–1470. doi:10.1016/j.bmcl.2004.12.080. PMID 15713408.
- ↑ Smith, B. M.; Smith, J. M.; Tsai, J. H.; Schultz, J. A.; Gilson, C. A.; Estrada, S. A.; Chen, R. R.; Park, D. M.; Prieto, E. B.; Gallardo, C. S.; Sengupta, D.; Dosa, P. I.; Covel, J. A.; Ren, A.; Webb, R. R.; Beeley, N. R. A.; Martin, M.; Morgan, M.; Espitia, S.; Saldana, H. R.; Bjenning, C.; Whelan, K. T.; Grottick, A. J.; Menzaghi, F.; Thomsen, W. J. (2008). "Discovery and Structure−Activity Relationship of (1R)-8-Chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a Selective Serotonin 5-HT2CReceptor Agonist for the Treatment of Obesity". Journal of Medicinal Chemistry. 51 (2): 305–313. doi:10.1021/jm0709034. PMID 18095642.
- ↑ "Lorcaserin New Drug Application". Drugs.com. 22 December 2009. Archived from the original on 3 March 2016.
- ↑ Andrew Pollack (16 September 2010). "F.D.A. Panel Urges Denial of Diet Drug". New York Times. Archived from the original on 22 August 2017.
- 1 2 "FDA Issues Complete Response Letter for Lorcaserin New Drug Application". 23 October 2010. Archived from the original on 24 October 2010.
- ↑ Arena Pharmaceuticals Announces Notification of Tentative September 16th FDA Advisory Committee Meeting to Review Lorcaserin for Weight Management Archived 2010-08-13 at the Wayback Machine., 2 June 2010
- ↑ Pollack, Andrew (16 September 2010). "F.D.A. Panel Rejects Diet Pill". The New York Times. Archived from the original on 17 July 2011.
- 1 2 "FDA approves lorcaserin, first weight-loss drug OK'd since 1999". Los Angeles Times. June 27, 2012. Archived from the original on June 29, 2012.
- ↑ "Archived copy". Archived from the original on 2012-09-28. Retrieved 2012-09-30.
- ↑ "New Diet Drug Lorcaserin Wins Vote From FDA Panel". webmd. 10 May 2012. Archived from the original on 12 May 2012.