''tert''-Amyl alcohol
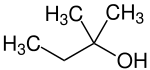 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Methylbutan-2-ol | |||
| Other names
2-Methyl-2-butanol tert-Amyl alcohol t-Amylol TAA tert-Pentyl alcohol 2-Methyl-2-butyl alcohol t-Pentylol Amylene hydrate Dimethylethylcarbinol | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1361351 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.827 | ||
| EC Number | 200-908-9 | ||
| KEGG | |||
| MeSH | tert-amyl+alcohol | ||
PubChem CID |
|||
| RTECS number | SC0175000 | ||
| UNII | |||
| UN number | 1105 | ||
| |||
| |||
| Properties | |||
| C5H12O | |||
| Molar mass | 88.15 g·mol−1 | ||
| Appearance | Colorless liquid | ||
| Odor | Camphorous | ||
| Density | 0.805 g/cm−3[1] | ||
| Melting point | −9 °C; 16 °F; 264 K | ||
| Boiling point | 101 to 103 °C; 214 to 217 °F; 374 to 376 K | ||
| 120 g·dm−3 | |||
| Solubility | soluble in water, benzene, chloroform, diethylether and ethanol[2] | ||
| log P | 1.095 | ||
| Vapor pressure | 1.6 kPa (at 20 °C) | ||
| −7.09×10−5 cm3/mol | |||
Refractive index (nD) |
1.405 | ||
| Viscosity | 4.4740 mPa·s (at 298.15 K)[1] | ||
| Thermochemistry | |||
Std molar entropy (S |
229.3 J K−1 mol−1 | ||
Std enthalpy of formation (ΔfH |
−380.0 to −379.0 kJ mol−1 | ||
Std enthalpy of combustion (ΔcH |
−3.3036 to −3.3026 MJ mol−1 | ||
| Hazards | |||
| Safety data sheet | hazard.com | ||
| GHS pictograms |   | ||
| GHS signal word | DANGER | ||
| H225, H315, H332, H335 | |||
| P210, P261 | |||
| NFPA 704 | |||
| Flash point | 19 °C (66 °F; 292 K) | ||
| 437 °C (819 °F; 710 K) | |||
| Explosive limits | 9% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
tert-Amyl alcohol (TAA), systematic name 2-methylbutan-2-ol (2M2B), is a branched pentanol.
Historically TAA has been used an anesthetic[3] and more recently it has also been used as a recreational drug similar to ethanol[4] because TAA is mostly a positive allosteric modulator for GABAA receptors[5][6] just like ethanol is.[7] This means that TAA causes calming effects within the central nervous system by interacting indirectly (allosterically) with GABAA receptors and enhances (positive effect) their activity.
TAA is a colorless liquid with a burning flavor[8] and a very unpleasant odor[9] which has been described as being similar to paraldehyde with a hint of camphor.[10] TAA can be produced via fermentation,[11] but it is primarily produced through other means.[3][12] TAA remains liquid at room temperature making it a useful alternative solvent to tert-butyl alcohol.
Production
Industrial
TAA is primarily produced via the hydration of 2-methyl-2-butene in the presence of an acidic catalyst.[12][3]
Natural occurrence
Fusel alcohols including TAA are grain fermentation byproducts and therefore trace amounts of TAA are present in many alcoholic beverages.[11] Trace levels of TAA have also been detected in various foods, including fried bacon,[13] cassava,[14] rooibos tea.[15]
History
Between about 1880 and 1950 TAA was used as an anesthetic with the contemporary name of amylene hydrate.[3] In 1930's[16] TAA was mainly used as a solvent for tribromoethanol (TBE), forming Avertin at a 0.5:1 volume ratio of TAA to TBE. TAA was rarely used as a sole hypnotic because of the existence of more efficient drugs.[3] Avertin is a brand-name for now discontinued TAA and TBE solution made by Winthrop Laboratories.[16]
Tertiary alcohols like TAA generally can not be oxidised to aldehyde or carboxylic acid[17] metabolites which are often toxic (e.g. acetaldehyde and formic acid from ethanol and methanol).
However, like other tertiary alcohol based anaesthetics (e.g. methylpentynol, ethchlorvynol) TAA was eventually superseded by safer and more effective agents. The use of TBE and TAA solution was also discontinued in humans in the late 1940s, as back then TBE was noted to be harmful for the liver just like chloroform, which was also used as an anesthetic at the time. TBE and TAA solution is still used as a short-acting anesthetic for laboratory mice and rats.[16]
Nowadays TAA has found use as a recreational drug.[4]
Use and effects
TAA produces euphoria, sedative, hypnotic, and anticonvulsant effects similar to ethanol through ingestion or inhalation.[18] When ingested, the effects of TAA may begin in about 30 minutes and can last up to 1–2 days.[19] 2–4 grams of TAA causes unconsciousness. About 100 g or 127 ml of ethanol induces similar level of unconsciousness.[20]
Overdose and toxicity
The smallest known dose of TAA that has killed a person is 30 ml.[19]
An overdose produces symptoms similar to alcohol poisoning and is a medical emergency due to the sedative/depressant properties which manifest in overdose as potentially lethal respiratory depression. The oral LD50 in rats is 1 g/kg. The subcutaneous LD50 in mice is 2.1 g/kg.[21]
Quick loss of consciousness, simultaneous respiratory and metabolic acidosis,[19] fast heartbeat, increased blood pressure, pupil constriction, coma, respiratory depression[22] and death may follow from an overdose. Somebody who has overdosed and suffers from respiratory depression may be kept alive by performing a tracheal intubation and then giving artificial respiration with pumps.[19]
Metabolism
In rats, TAA is primarily metabolized via glucuronidation, as well as by oxidation to 2-methyl-2,3-butanediol. It is likely that the same path is followed in humans,[23] though older sources suggest TAA is excreted unchanged.[3]

The use of TAA cannot be detected with general ethanol tests or other ordinary drug tests. Its use can be detected from a blood or a urine sample by using gas chromatography–mass spectrometry for up to 48 hours after consumption.[22]
See also
References
- 1 2 Lomte, S.B.; Bawa, M.J.; Lande, M.K.; Arbad, B.R. (2009). "Densities and Viscosities of Binary Liquid Mixtures of 2-Butanone with Branched Alcohols at (293.15 to 313.15) K". Journal of Chemical & Engineering Data. 54: 127–130. doi:10.1021/je800571y.
- ↑ Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2014). "Section 3 - Physical Constants of Organic Compounds". CRC Handbook of Chemistry and Physics, 95th Edition (95th ed.). Boca Raton, Florida: CRC Press. ISBN 9781482208689. OCLC 908078665.
- 1 2 3 4 5 6 Adriani, John (1962). The Chemistry and Physics of Anesthesia (2nd ed.). Illinois: Thomas Books. pp. 273–274. ISBN 9780398000110.
- 1 2 Rusiecka, Izabela; Gągało, Iwona; Anand, Jacek Sein; Schetz, Daria; Waldman, Wojciech (October 2016). "Drinking "Vodka" or vodka – This is a question". Toxicology In Vitro. 36: 66–70. doi:10.1016/j.tiv.2016.07.009. ISSN 1879-3177. PMID 27448500.
- ↑ Martin, J (2004). "Influence of oxygenated fuel additives and their metabolites on γ-aminobutyric acidA (GABAA) receptor function in rat brain synaptoneurosomes". Toxicology Letters. 147 (3): 209–217. doi:10.1016/j.toxlet.2003.10.024.
- ↑ Martin, Joseph V.; Bilgin, Nesli M.; Iba, M. Michael (2002). "Influence of oxygenated fuel additives and their metabolites on the binding of a convulsant ligand of the γ-aminobutyric acidA (GABAA) receptor in rat brain membrane preparations". Toxicology Letters. 129 (3): 219–226. doi:10.1016/s0378-4274(02)00020-6.
- ↑ Lobo, Ingrid A.; Harris, R. Adron (2008). "GABAA receptors and alcohol" (Accepted manuscript). Pharmacology Biochemistry and Behavior. 90 (1): 90–94. doi:10.1016/j.pbb.2008.03.006. PMC 2574824. PMID 18423561.
- ↑ O'Neil, Maryadele J., ed. (2006). The Merck index (14th ed.). Whitehouse Station, NJ: Merck. p. 1232. ISBN 9780911910001. OCLC 70882070.
- ↑ Lewis, R. J. (2001). Hawley's Condensed Chemical Dictionary. New York, NY: John Wiley & Sons, Inc. p. 70.
- ↑ Yandell, D. W.; et al. (1888). "Amylene hydrate, a new hypnotic". The American Practitioner and News. Louisville, KY. 5: 88–98.
- 1 2 Gould, George M.; Scott, Richard J. E. (1919). The Practitioner's Medical Dictionary. P. Blakiston's. p. 50. Retrieved 2018-07-27.
- 1 2 Papa, Anthony J. (2004). "Amyl Alcohols". Kirk–Othmer Encyclopedia of Chemical Technology (5th ed.). Hoboken, N.J.: Wiley-Interscience. doi:10.1002/0471238961.0113251216011601.a01.pub2. ISBN 9780471238966.
- ↑ Ho, C.-T.; Lee, K.-N.; Jin, Q.-Z. (1983). "Isolation and identification of volatile flavor compounds in fried bacon". Journal of Agricultural and Food Chemistry. 31 (2): 336. doi:10.1021/jf00116a038. ISSN 0021-8561.
- ↑ Dougan, J.; Robinson, J. M.; Sumar, S.; Howard, G. E.; Coursey, D. G. (1983). "Some flavouring constituents of cassava and of processed cassava products". Journal of the Science of Food and Agriculture. 34 (8): 874. doi:10.1002/jsfa.2740340816. ISSN 1097-0010.
- ↑ Habu, Tsutomu; Flath, Robert A.; Mon, T. Richard; Morton, Julia F. (1 March 1985). "Volatile components of Rooibos tea (Aspalathus linearis)". Journal of Agricultural and Food Chemistry. 33 (2): 249–254. doi:10.1021/jf00062a024. ISSN 0021-8561.
- 1 2 3 Meyer, Robert E.; Fish, Richard E. (November 2005). "A review of tribromoethanol anesthesia for production of genetically engineered mice and rats". Lab Animal. 34 (10): 47–52. doi:10.1038/laban1105-47. ISSN 0093-7355. PMID 16261153.
- ↑ Carey, Francis (2000). Organic Chemistry (4 ed.). ISBN 978-0072905014. Archived from the original on 2017-07-07. Retrieved 2013-02-05.
- ↑ Lewis, Robert Alan (1998). Lewisʼ Dictionary of Toxicology. Boca Raton, Florida: CRC Press. p. 45. ISBN 978-1566702232. OCLC 35269968.
- 1 2 3 4 "2-METHYL-2-BUTANOL - National Library of Medicine HSDB Database". www.toxnet.nlm.nih.gov. Archived from the original on 2018-03-08. Retrieved 2018-04-08.
- ↑ Brandenberger, Hans; Maes, Robert A. A. (1997). Analytical Toxicology for Clinical, Forensic, and Pharmaceutical Chemists. Berlin: W. de Gruyter. ISBN 978-3110107319. OCLC 815506841.
- ↑ Soehring, K.; Frey, H.H.; Endres, G. (1955). "Relations between constitution and effect of tertiary alcohols". Arzneimittel-Forschung. 5 (4): 161–165. PMID 14389140.
- 1 2 Anand, Jacek Sein; Gieroń, Joanna; Lechowicz, Wojciech; Schetz, Daria; Kała, Maria; Waldman, Wojciech (September 2014). "Acute intoxication due to tert-amyl alcohol—a case report". Forensic Science International. 242: e31–e33. doi:10.1016/j.forsciint.2014.07.020. ISSN 1872-6283. PMID 25112153.
- ↑ Collins, A. S.; Sumner, S. C.; Borghoff, S. J.; Medinsky, M. A. (1999). "A physiological model for tert-amyl methyl ether and tert-amyl alcohol: Hypothesis testing of model structures". Toxicological Sciences. 49 (1): 15–28. doi:10.1093/toxsci/49.1.15. PMID 10367338.