Olopatadine
 | |
| Clinical data | |
|---|---|
| Trade names | Patanol and others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602025 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Ophthalmic, intranasal, oral |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 3 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.133.834 |
| Chemical and physical data | |
| Formula | C21H23NO3 |
| Molar mass | 337.412 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Olopatadine is an antihistamine (as well as anticholinergic and mast cell stabilizer), sold as a prescription eye drop manufactured by Alcon in one of three strengths: 0.7% solution or Pazeo in the United States, 0.2% solution or Pataday (also called Patanol S in some countries), and 0.1% or Patanol (also called Opatanol in some countries; Olopat in India). It is used to treat itching associated with allergic conjunctivitis (eye allergies).[1] A steroid-free[2] nasal spray formulation is sold as Patanase, which was approved by the FDA on April 15, 2008.[3] It is also available as an oral tablet in Japan under the tradename Allelock, manufactured by Kyowa Hakko Kogyo.[4]
The usual dose for Patanol is 1 drop in each affected eye 2 times per day, with 6 to 8 hours between doses. Both Pazeo and Pataday are dosed 1 drop in each eye daily.
There is potential for olopatadine as a treatment modality for steroid rebound (red skin syndrome).[5]
Olopatadine was developed by Kyowa Hakko Kogyo.[6]
Side Effects
Some known side effects include headache (7% of occurrence), eye burning and/or stinging (5%), blurred vision, dry eyes, foreign body sensation, hyperemia, keratitis, eyelid edema, pruritus, asthenia, sore throat (pharyngitis), rhinitis, sinusitis, taste perversion, and vomiting.
Chemistry
Synthesis
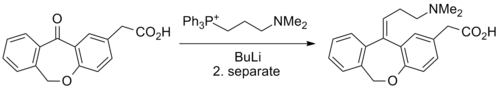
Pharmacology
Pharmacodynamics
Olopatadine acts as a selective antagonist of the histamine H1 receptor, thus stabilizing mast cells and inhibiting histamine release.
References
- ↑ Castillo M, Scott NW, Mustafa MZ, Mustafa MS, Azuara-Blanco A (2015). "Topical antihistamines and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis". Cochrane Database Syst Rev. 6 (6): CD009566. doi:10.1002/14651858.CD009566.pub2. PMID 26028608.
- ↑ How PATANASE® Nasal Spray Works
- ↑ Drugs.com, Alcon's Patanase Nasal Spray Approved by FDA for Treatment of Nasal Allergy Symptoms
- ↑ Kyowa Hakko Kogyo Co., Ltd. (2007). "ALLELOCK Tablets 2.5 & ALLELOCK Tablets 5 (English)" (PDF). Retrieved 2008-08-10.
- ↑ Tamura T; Matsubara M; Hasegawa K; Ohmori K; Karasawa A. (2005). "Olopatadine hydrochloride suppresses the rebound phenomenon after discontinuation of treatment with a topical steroid in mice with chronic contact hypersensitivity". Clin Exp Allergy. 35 (1): 97–103. doi:10.1111/j.1365-2222.2005.02147.x. PMID 15649273.
- ↑ Kyowa Hakko Kogyo Co., Ltd. (2002). "Company History". Company Information. Kyowa Hakko Kogyo Co., Ltd. Retrieved 16 September 2010.
- ↑ Ueno, K.; Kubo, S.; Tagawa, H.; Yoshioka, T.; Tsukada, W.; Tsubokawa, M.; Kojima, H.; Kasahara, A. (1976). "6,11-Dihydro-11-oxodibenz[b,e]oxepinacetic acids with potent antiinflammatory activity". Journal of Medicinal Chemistry. 19 (7): 941–946. doi:10.1021/jm00229a017.