Pitolisant
 | |
| Clinical data | |
|---|---|
| Trade names | Wakix |
| Synonyms | Tiprolisant; Ciproxidine; BF2.649 |
| License data | |
| Routes of administration | Oral |
| Drug class | Histamine H3 receptor inverse agonists |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
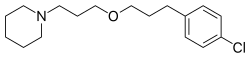
| Formula | C17H26ClNO |
| Molar mass | 295.851 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Pitolisant (INN), also known as tiprolisant (USAN), and sold under the brand name Wakix, is a potent and selective inverse agonist of the histamine H3 receptor (Ki = 0.16 nM) which was approved for the treatment of narcolepsy in 2016.[1][2][3][4] It is also currently in phase III clinical trials for the treatment of hypersomnia.[1]
Pitolisant was developed by Jean-Charles Schwartz, Walter Schunack, and colleagues after the former discovered the H3 receptor.[5] It was the first H3 receptor inverse agonist to be tested in humans or introduced for clinical use.[5]
References
- 1 2 http://adisinsight.springer.com/drugs/800029451
- ↑ Syed YY (2016). "Pitolisant: First Global Approval". Drugs. 76 (13): 1313–8. doi:10.1007/s40265-016-0620-1. PMID 27438291.
- ↑ Schlicker E, Kathmann M (2017). "Role of the Histamine H3 Receptor in the Central Nervous System". Handb Exp Pharmacol. 241: 277–299. doi:10.1007/164_2016_12. PMID 27787717.
- ↑ Calik MW (2017). "Update on the treatment of narcolepsy: clinical efficacy of pitolisant". Nat Sci Sleep. 9: 127–133. doi:10.2147/NSS.S103462. PMC 5414617. PMID 28490912.
- 1 2 Schwartz JC (2011). "The histamine H3 receptor: from discovery to clinical trials with pitolisant". Br. J. Pharmacol. 163 (4): 713–21. doi:10.1111/j.1476-5381.2011.01286.x. PMC 3111674. PMID 21615387.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.