Clonidine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˈklɒnədiːn/ |
| Trade names | Catapres, Kapvay, Nexiclon, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682243 |
| License data | |
| Pregnancy category | |
| Routes of administration | By mouth, epidural, IV, transdermal, topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 75–95% (oral), 60–70% (transdermal)[1] |
| Protein binding | 20–40%[2] |
| Metabolism | Hepatic to inactive metabolites,[2] 2/3 CYP2D6 |
| Elimination half-life | IR: 12–16 hours,[3] 48 hours for repeated dosing[1] |
| Excretion | Urine (72%)[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.021.928 |
| Chemical and physical data | |
| Formula | C9H9Cl2N3 |
| Molar mass | 230.093 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Clonidine is a medication that is used to treat high blood pressure, attention deficit hyperactivity disorder, anxiety disorders, tic disorders, withdrawal (from either alcohol, opioids, or smoking), migraine, menopausal flushing, diarrhea, and certain pain conditions.[4] It is marketed under many brand names.
The adverse effects include sedation, dry mouth, and low blood pressure.[2]
Clonidine treats high blood pressure by stimulating α2 receptors in the brain stem, which decreases peripheral vascular resistance, lowering blood pressure. Clonidine also may cause bradycardia, probably by increasing signaling through the vagus nerve. When given intravenously, clonidine temporarily increases blood pressure by stimulating postsynaptic α2 receptors in smooth muscle in the lining of blood vessels. This does not occur when clonidine is given by mouth or transdermally.[5]:201–203
Medical uses
Clonidine was introduced in 1966.[8] It was first used as a hypertension treatment under the trade name of Catapres.[9] The US Food and Drug Administration (FDA) has approved clonidine for the treatment of attention deficit hyperactivity disorder (ADHD), under the trade name of Kapvay alone or with stimulants in 2010, for pediatric patients aged 6–17 years.[2][10] It was later approved for adults. In Australia, clonidine is an accepted but not approved use for ADHD by the TGA.[11] Clonidine along with methylphenidate has been studied for treatment of ADHD.[12][13][14] While not as effective as methylphenidate in treating ADHD, Clonidine does offer some benefit;[15] it can also be useful in combination with stimulant medications.[16] Some studies show clonidine more sedating than guanfacine, which may be better at bed time along with an arousing stimulant at morning.[17][18] Clonidine can be used in the treatment of Tourette syndrome (specifically for tics).[19]
Clonidine may be used to ease withdrawal symptoms associated with the long-term use of narcotics, alcohol, benzodiazepines and nicotine (smoking).[20] It can alleviate opioid withdrawal symptoms by reducing the sympathetic nervous system response such as tachycardia and hypertension, as well as reducing sweating, hot and cold flashes, and general restlessness.[21] It may also be helpful in aiding smokers to quit.[22] The sedation effect is also useful. However, its side effects can include insomnia, thus exacerbating an already common feature of opioid withdrawal.[23]
Clonidine also has several off-label uses, and has been prescribed to treat psychiatric disorders including stress, sleep disorders, and hyperarousal caused by post-traumatic stress disorder, borderline personality disorder, and other anxiety disorders.[24][25][26][27][28][29][30][31] Clonidine is also a mild sedative, and can be used as premedication before surgery or procedures.[32] Its epidural use for pain during heart attack, postoperative and intractable pain has also been studied extensively.[33] Clonidine has also been suggested as a treatment for rare instances of dexmedetomidine withdrawal.[34] Clonidine can be used in restless legs syndrome.[35] It can also be used to treat facial flushing and redness associated with rosacea.[36] It has also been successfully used topically in a clinical trial as a treatment for diabetic neuropathy.[37] Clonidine can also be used for migraine headaches and hot flashes associated with menopause.[38][39] Clonidine has also been used to treat diarrhea associated with irritable bowel syndrome, fecal incontinence, diabetes, withdrawal-associated diarrhea, intestinal failure, neuroendocrine tumors and cholera.[40]
Clonidine suppression test
The reduction in circulating norepinephrine by clonidine was used in the past as an investigatory test for phaeochromocytoma, which is a catecholamine-synthesizing tumour, usually found in the adrenal medulla.[41] In a clonidine suppression test plasma catecholamine levels are measured before and 3 hours after a 0.3 mg oral test dose has been given to the patient. A positive test occurs if there is no decrease in plasma levels.[41]
Pregnancy
Clonidine is classed by the FDA as pregnancy category C. It is classified by the TGA of Australia as pregnancy category B3, which means that it has shown some detrimental effects on fetal development in animal studies, although the relevance of this to human beings is unknown.[42] Clonidine can pass into breast milk and may harm a nursing baby; caution is warranted in women who are pregnant, planning to become pregnant, or are breastfeeding.[43]
Adverse effects
The principal adverse effects of clonidine are sedation, dry mouth, and hypotension (low blood pressure).[2]
Adverse effects by frequency[42][44]
Very common (>10% frequency):
- Dizziness
- Orthostatic hypotension
- Somnolence (dose-dependent)
- Xerostomia (dry mouth)
- Headache (dose-dependent)
- Fatigue
- Skin reactions (if given transdermally)
- Hypotension
Common (1-10% frequency):
- Anxiety
- Constipation
- Sedation (dose-dependent)
- Nausea/vomiting
- Malaise
- Abnormal LFTs
- Rash
- Weight gain/loss
- Pain below the ear (from salivary gland)
- Erectile dysfunction
Uncommon (0.1-1% frequency):
- Delusional perception
- Hallucination
- Nightmare
- Paresthesia
- Sinus bradycardia
- Raynaud's phenomenon
- Pruritus
- Urticaria
Rare (<0.1% frequency):
- Gynaecomastia
- Impaired ability to cry
- Atrioventricular block
- Nasal dryness
- Colonic pseudo-obstruction
- Alopecia
- Hyperglycaemia
Withdrawal
Clonidine suppresses sympathetic outflow resulting in lower blood pressure, but sudden discontinuation can cause rebound hypertension due to a rebound in sympathetic outflow.[4]
Clonidine therapy should generally be gradually tapered when discontinuing therapy to avoid rebound effects from occurring. Treatment of clonidine withdrawal hypertension depends on the severity of the condition. Reintroduction of clonidine for mild cases, alpha and beta blockers for more urgent situations. Beta blockers never should be used alone to treat clonidine withdrawal as alpha vasoconstriction would still continue.[45][46]
Pharmacology
Mechanism of action
| Receptor | Ki (nM)[47] |
|---|---|
| Alpha-1A adrenergic receptor | 316.23 |
| Alpha-1B adrenergic receptor | 316.23 |
| Alpha-1D adrenergic receptor | 125.89 |
| Alpha-2A adrenergic receptor | 42.92 |
| Alpha-2B adrenergic receptor | 106.31 |
| Alpha-2C adrenergic receptor | 233.1 |
| The Ki refers to a drug's affinity for a receptor. The smaller the Ki, the higher the affinity for that receptor.[48] | |
High blood pressure
Clonidine treats high blood pressure by stimulating α2 receptors in the brain stem, which decreases peripheral vascular resistance, lowering blood pressure. It has specificity towards the presynaptic α2 receptors in the vasomotor center in the brainstem. This binding has a sympatholytic effect, suppresses release of norepinephrine, ATP, renin, and neuropeptide Y which if released would increase vascular resistance.[5]:201–203
Clonidine also acts as an agonist at imidazoline-1 (I1) receptors in the brain, and it is hypothesized that this effect may contribute to reducing blood pressure by reducing signaling in the sympathetic nervous system, but this effect acts upstream of the central α2 agonist effect of clonidine.[5]:201–203[49]
Clonidine also may cause bradycardia, probably by increasing signaling through the vagus nerve. When given intravenously, clonidine can temporarily increase blood pressure by stimulating α1 receptors in smooth muscles in blood vessels.[50] This hypertensive effect is not usual when clonidine is given by mouth or by the transdermal route.[5]:201–203
Attention deficit hyperactivity disorder
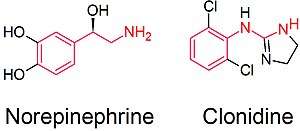
In the setting of attention deficit hyperactivity disorder (ADHD), clonidine's molecular mechanism of action occurs due to its agonism at the alpha-2A adrenergic receptor, the subtype of the alpha-2 adrenergic receptor that is most principally found in the brain. Within the brain, the alpha-2A adrenergic receptors are found within the prefrontal cortex (PFC), among other areas. The alpha-2A adrenergic receptors are found on the presynaptic cleft of a given neuron, and, when activated by an agonist, the effect on downstream neurons is inhibitory. The inhibition is accomplished by preventing the secretion of the neurotransmitter norepinephrine. Thus, clonidine's agonism on alpha-2A adrenergic receptors in the PFC inhibits the action of downstream neurons by preventing the secretion of norepinephrine.[51]
This mechanism is similar to the brain's physiological inhibition of PFC neurons by the locus ceruleus (LC), which secretes norepinephrine into the PFC. Although norepinephrine can also bind to target adrenergic receptors on the downstream neuron (otherwise inducing a stimulatory effect), norepinephrine also binds to alpha-2A adreneric receptors (akin to clonidine's mechanism of action), inhibiting the release of norepinephrine by that neuron and inducing an inhibitory effect. Because the PFC is required for working memory and attention, it is thought that clonidine's inhibition of PFC neurons helps to eliminate irrelevant attention (and subsequent behaviors), improving the person's focus and correcting deficits in attention.[51]
Growth hormone test
Clonidine stimulates release of growth hormone releasing hormone from the hypothalamus, which in turn stimulates pituitary release of growth hormone.[52] This effect has been used as part of a "growth hormone test," which can assist with diagnosing growth hormone deficiency in children.[53]
Pharmacokinetics
After being ingested, clonidine is absorbed into the blood stream rapidly and nearly completely, with peak concentrations in human plasma occurring within 60–90 minutes.[54] Clonidine is fairly lipid soluble with the logarithm of its partition coefficient (log P) equal to 1.6;[55][54] to compare, the optimal log P to allow a drug that is active in the human central nervous system to penetrate the blood brain barrier is 2.0.[56] Less than half of the absorbed portion of an orally administered dose will be metabolized by the liver into inactive metabolites, with roughly the other half being excreted unchanged by the kidneys.[54] About one-fifth of an oral dose will not be absorbed, and is thus excreted in the feces.[54] The half-life of clonidine varies widely, with estimates between 6 and 23 hours, and is greatly affected by and prolonged in the setting of poor kidney function.[54]
Brand names
As of June 2017 clonidine was marketed under many brand names worldwide: Arkamin, Aruclonin, Atensina, Catapin, Catapres, Catapresan, Catapressan, Chianda, Chlofazoline, Chlophazolin, Clonid-Ophtal, Clonidin, Clonidina, Clonidinã, Clonidine, Clonidine hydrochloride, Clonidinhydrochlorid, Clonidini, Clonidinum, Clonigen, Clonistada, Clonnirit, Clophelinum, Dixarit, Duraclon, Edolglau, Haemiton, Hypodine, Hypolax, Iporel, Isoglaucon, Jenloga, Kapvay, Klofelino, Kochaniin, Melzin, Menograine, Normopresan, Paracefan, Pinsanidine, Run Rui, and Winpress.[57] It was marketed as a combination drug with chlortalidone as Arkamin-H, Bemplas, Catapres-DIU, and Clorpres, and in combination with bendroflumethiazide as Pertenso.[57]
References
- 1 2 Lowenthal, DT; Matzek, KM; MacGregor, TR (May 1988). "Clinical pharmacokinetics of clonidine". Clinical Pharmacokinetics. 14 (5): 287–310. doi:10.2165/00003088-198814050-00002. PMID 3293868.
- 1 2 3 4 5 6 "clonidine (Rx) - Catapres, Catapres-TTS, more." Medscape Reference. WebMD. Retrieved 10 November 2013.
- ↑ "Kapvay". RxList.
- 1 2 Brayfield, A, ed. (13 January 2014). "Clonidine". Martindale: The Complete Drug Reference. London, UK: Pharmaceutical Press. Retrieved 28 June 2014.
- 1 2 3 4 Westfall, Thomas C.; Macarthur, Heather; Westfall, David P (2017). "Chapter 12:Adrenergic Agonists and Antagonists". In Brunton, Laurence; Knollmann, Bjorn; Hilal-Dandan, Randa. Goodman and Gilman's The Pharmacological Basis of Therapeutics (13th ed.). McGraw-Hill Education / Medical. ISBN 9781259584732.
- ↑ Neil, MJ (November 2011). "Clonidine: clinical pharmacology and therapeutic use in pain management". Current Clinical Pharmacology. 6 (4): 280–7. doi:10.2174/157488411798375886. PMID 21827389.
- ↑ Stähle, Helmut (June 2000). "A historical perspective: development of clonidine". Best Practice & Research Clinical Anaesthesiology. 14 (2): 237–246. doi:10.1053/bean.2000.0079.
- ↑ Stähle, Helmut (June 2000). "A historical perspective: development of clonidine". Best Practice & Research Clinical Anaesthesiology. 14 (2): 237–246. doi:10.1053/bean.2000.0079.
- ↑ "Clonidine: Drug Uses, Dosage & Side Effects - Drugs.com". Drugs.com. Retrieved 2017-12-10.
- ↑ "FDA Approves Extended-Release Clonidine for Pediatric ADHD". WebMD LLC.
- ↑ Rossi, S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ↑ Palumbo, DR; Sallee, FR; Pelham WE, Jr; Bukstein, OG; Daviss, WB; McDermott, MP (February 2008). "Clonidine for attention-deficit/hyperactivity disorder: I. Efficacy and tolerability outcomes". Journal of the American Academy of Child and Adolescent Psychiatry. 47 (2): 180–8. doi:10.1097/chi.0b013e31815d9af7. PMID 18182963.
- ↑ Daviss, WB; Patel, NC; Robb, AS; McDermott, MP; Bukstein, OG; Pelham WE, Jr; Palumbo, D; Harris, P; Sallee, FR (February 2008). "Clonidine for attention-deficit/hyperactivity disorder: II. ECG changes and adverse events analysis". Journal of the American Academy of Child and Adolescent Psychiatry. 47 (2): 189–98. doi:10.1097/chi.0b013e31815d9ae4. PMID 18182964.
- ↑ Kornfield R, Watson S, Higashi AS, Conti RM, Dusetzina SB, Garfield CF, Dorsey ER, Huskamp HA, Alexander GC (April 2013). "Impact of FDA Advisories on Pharmacologic Treatment of Attention Deficit Hyperactivity Disorder". Psychiatric Services. 64 (4): 339–46. doi:10.1176/appi.ps.201200147. PMC 4023684. PMID 23318985.
- ↑ PALUMBO, DONNA R.; SALLEE, FLOYD R.; PELHAM, WILLIAM E.; BUKSTEIN, OSCAR G.; DAVISS, W. BURLESON; McDERMOTT, MICHAEL P. (February 2008). "Clonidine for Attention-Deficit/Hyperactivity Disorder: I. Efficacy and Tolerability Outcomes". Journal of the American Academy of Child & Adolescent Psychiatry. 47 (2): 180–188. doi:10.1097/chi.0b013e31815d9af7. PMID 18182963.
- ↑ Kollins, Scott H.; Jain, Rakesh; Brams, Matthew; Segal, Scott; Findling, Robert L.; Wigal, Sharon B.; Khayrallah, Moise (2011-06-01). "Clonidine Extended-Release Tablets as Add-on Therapy to Psychostimulants in Children and Adolescents With ADHD". Pediatrics. 127 (6): e1406–e1413. doi:10.1542/peds.2010-1260. ISSN 0031-4005. PMC 3387872. PMID 21555501.
- ↑ "Guanfacine, But Not Clonidine, Improves Planning and Working Memory Performance in Humans". Neuropsychopharmacology. Retrieved August 1, 2014.
- ↑ "Clonidine and Guanfacine IR vs ER: Old Drugs With "New" Formulations". Mental Health Clinician. Retrieved August 1, 2014.
- ↑ Egolf, A; Coffey, BJ (February 2014). "Current pharmacotherapeutic approaches for the treatment of Tourette syndrome". Drugs of Today. 50 (2): 159–79. doi:10.1358/dot.2014.50.2.2097801. PMID 24619591.
- ↑ Fitzgerald, PJ (October 2013). "Elevated Norepinephrine may be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine". Substance Abuse. 7: 171–83. doi:10.4137/SART.S13019. PMC 3798293. PMID 24151426.
- ↑ Giannini, AJ (1997). Drugs of Abuse (2nd ed.). Los Angeles: Practice Management Information.
- ↑ Gourlay, SG; Stead, LF; Benowitz, NL (2004). "Clonidine for smoking cessation". The Cochrane Database of Systematic Reviews (3). CD000058. doi:10.1002/14651858.CD000058.pub2. PMID 15266422.
- ↑ Giannini, AJ; Extein, I; Gold, MS; Pottash, ALC; Castellani, S (1983). "Clonidine in mania". Drug Development Research. 3 (1): 101–105. doi:10.1002/ddr.430030112.
- ↑ van der Kolk, BA (September–October 1987). "The drug treatment of post-traumatic stress disorder". Journal of Affective Disorders. 13 (2): 203–13. doi:10.1016/0165-0327(87)90024-3. PMID 2960712.
- ↑ Sutherland, SM; Davidson, JR (June 1994). "Pharmacotherapy for post-traumatic stress disorder". The Psychiatric Clinics of North America. 17 (2): 409–23. PMID 7937367.
- ↑ Southwick, SM; Bremner, JD; Rasmusson, A; Morgan CA, 3rd; Arnsten, A; Charney, DS (November 1999). "Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder". Biological Psychiatry. 46 (9): 1192–204. doi:10.1016/S0006-3223(99)00219-X. PMID 10560025.
- ↑ Strawn, JR; Geracioti, TD, Jr (2008). "Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder". Depression and Anxiety. 25 (3): 260–71. doi:10.1002/da.20292. PMID 17354267.
- ↑ Boehnlein, JK; Kinzie, JD (March 2007). "Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin". Journal of Psychiatric Practice. 13 (2): 72–8. doi:10.1097/01.pra.0000265763.79753.c1. PMID 17414682.
- ↑ Huffman, JC; Stern, TA (2007). "Neuropsychiatric consequences of cardiovascular medications". Dialogues in Clinical Neuroscience. 9 (1): 29–45. PMC 3181843. PMID 17506224.
- ↑ Najjar, F; Weller, RA; Weisbrot, J; Weller, EB (April 2008). "Post-traumatic stress disorder and its treatment in children and adolescents". Current Psychiatry Reports. 10 (2): 104–8. doi:10.1007/s11920-008-0019-0. PMID 18474199.
- ↑ Ziegenhorn, AA; Roepke, S; Schommer, NC; Merkl, A; Danker-Hopfe, H; Perschel, FH; Heuser, I; Anghelescu, IG; Lammers, CH (April 2009). "Clonidine improves hyperarousal in borderline personality disorder with or without comorbid posttraumatic stress disorder: a randomized, double-blind, placebo-controlled trial". Journal of Clinical Psychopharmacology. 29 (2): 170–3. doi:10.1097/JCP.0b013e31819a4bae. PMID 19512980.
- ↑ Fazi, L; Jantzen, EC; Rose, JB; Kurth, CD; Watcha, MF (2001). "A comparison of oral clonidine and oral midazolam as preanesthetic medications in the pediatric tonsillectomy patient" (PDF). Anesthesia and Analgesia. 92 (1): 56–61. doi:10.1097/00000539-200101000-00011. PMID 11133600.
- ↑ Patel, SS; Dunn, CJ; Bryson, HM (1996). "Epidural clonidine: a review of its pharmacology and efficacy in the management of pain during labour and postoperative and intractable pain". CNS Drugs. 6 (6): 474–497. doi:10.2165/00023210-199606060-00007.
- ↑ Kukoyi A, Coker S, Lewis L, Nierenberg D (January 2013). "Two cases of acute dexmedetomidine withdrawal syndrome following prolonged infusion in the intensive care unit: Report of cases and review of the literature". Human and Experimental Toxicology. 32 (1): 107–110. doi:10.1177/0960327112454896. PMID 23111887. Retrieved 1 March 2013.
- ↑ "Treatment and Management of RLS". www.medscape.org. WebMD LLC. Retrieved 3 October 2018.
- ↑ Blount, BW; Pelletier, AL (2002). "Rosacea: A Common, Yet Commonly Overlooked, Condition". American Family Physician. 66 (3): 435–441. PMID 12182520.
- ↑ Campbell, CM; Kipnes, MS; Stouch, BC; Brady, KL; Kelly, M; Schmidt, WK; Petersen, KL; Rowbotham, MC; Campbell, JN (September 2012). "Randomized control trial of topical clonidine for treatment of painful diabetic neuropathy". Pain. 153 (9): 1815–1823. doi:10.1016/j.pain.2012.04.014. PMC 3413770. PMID 22683276.
- ↑ "Clonidine Oral Uses". WebMD.
- ↑ "Clonidine". Drugs.com.
- ↑ Fragkos, Konstantinos C.; Zárate-Lopez, Natalia; Frangos, Christos C. (2016-01-25). "What about clonidine for diarrhoea? A systematic review and meta-analysis of its effect in humans". Therapeutic Advances in Gastroenterology. 9 (3). 1756283X15625586. doi:10.1177/1756283X15625586. ISSN 1756-283X. PMC 4830099.
- 1 2 Eisenhofer, G; Goldstein, DS; Walther, MM; et al. (2003). "Biochemical diagnosis of pheochromocytoma: how to distinguish true- from false-positive test results". Journal of Clinical Endocrinology & Metabolism. 88 (6): 2656–2666. doi:10.1210/jc.2002-030005. PMID 12788870.
- 1 2 "CATAPRES® 150 TABLETS CATAPRES® AMPOULES" (PDF). TGA eBusiness Services. Boehringer Ingelheim Pty Limited. 28 February 2013. Retrieved 27 November 2013.
- ↑ "Clonidine". Prescription Marketed Drugs. www.drugsdb.eu.
- ↑ "Clonidine 25 mcg Tablets BP - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Sandoz Limited. 2 August 2012. Retrieved 27 November 2013.
- ↑ Parker, K; Brunton, L; Goodman, LS; Lazo, JS; Gilman, A (2006). Goodman & Gilman's - the pharmacological basis of therapeutics. New York: McGraw-Hill. pp. 854–855. ISBN 0-07-142280-3.
- ↑ Vitiello, B. (2008). "Understanding the Risk of Using Medications for ADHD with Respect to Physical Growth and Cardiovascular Function". Child Adolesc Psychiatr Clin N Am. 17 (2): 459–474, xi. doi:10.1016/j.chc.2007.11.010. PMC 2408826. PMID 18295156.
- ↑ Roth, BL; Driscol, J (12 January 2011). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Archived from the original on 8 November 2013. Retrieved 25 November 2013.
- ↑ Terry Kenakin (2009). "Ligand-Receptor Binding and Tissue Response". In Hacker, Miles; Messer, William; Bachmann, Kenneth. Pharmacology. Elsevier. p. 65. ISBN 9780123695215.
- ↑ Reis, D. J.; Piletz, J. E. (1997). "The imidazoline receptor in control of blood pressure by clonidine and drugs" (pdf). American Journal of Physiology. 273 (5): R1569–R1571. PMID 9374795.
- ↑ Giovannitti, Joseph A.; Thoms, Sean M.; Crawford, James J. (2015). "Alpha-2 Adrenergic Receptor Agonists: A Review of Current Clinical Applications". Anesthesia Progress. 62 (1): 31–38. doi:10.2344/0003-3006-62.1.31. ISSN 0003-3006. PMC 4389556. PMID 25849473.
- 1 2 3 Cinnamon Bidwell, L; Dew, RE; Kollins, SH (October 2010). "Alpha-2 adrenergic receptors and attention-deficit/hyperactivity disorder". Current psychiatry reports. 12 (5): 366–73. doi:10.1007/s11920-010-0136-4. PMC 3676929. PMID 20652773.
- ↑ Low LC (1991). "Growth hormone-releasing hormone: clinical studies and therapeutic aspects". Neuroendocrinology. 53 Suppl 1: 37–40. doi:10.1159/000125793. PMID 1901390.
- ↑ "Growth Hormone Test". www.cincinnatichildrens.org. Cincinnati Children's Hospital Medical Center. Retrieved 13 October 2018.
- 1 2 3 4 5 Khan, ZP; Ferguson, CN; Jones, RM (February 1999). "alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role". Anaesthesia. 54 (2): 146–65. PMID 10215710.
- ↑ Foye's principles of medicinal chemistry (6th ed.). Philadelphia: Lippincott Williams & Wilkins. 2008. p. 403. ISBN 9780781768795.
- ↑ Pajouhesh, H; Lenz, GR (October 2005). "Medicinal chemical properties of successful central nervous system drugs". NeuroRx. 2 (4): 541–53. doi:10.1602/neurorx.2.4.541. PMID 16489364.
- 1 2 "Clonidine brand names". Drugs.com. Retrieved 16 June 2017.
External links
| Wikimedia Commons has media related to Clonidine. |
- U.S. National Library of Medicine: Drug Information Portal - Clonidine
- Alpha-2 agonists in ADHD