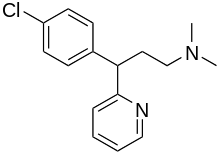
Chlorphenamine
 | |
| Clinical data | |
|---|---|
| Trade names | Chlor-Trimeton; Piriton |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682543 |
| Pregnancy category | |
| Routes of administration | By mouth, IV, IM, SC |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 25 to 50% |
| Protein binding | 72% |
| Metabolism | Hepatic (CYP2D6) |
| Elimination half-life | 13.9–43.4 hours[1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.004.596 |
| Chemical and physical data | |
| Formula | C16H19ClN2 |
| Molar mass | 274.79 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 0.55 g/100 mL, liquid mg/mL (20 °C) |
| |
| |
| | |
Chlorphenamine (also known as chlorpheniramine, CP, or CPM) is a first-generation antihistamine used in the prevention of the symptoms of allergic conditions such as rhinitis and urticaria. Its sedative effects are relatively weak compared to other first-generation antihistamines. Chlorphenamine is one of the most commonly used antihistamines in small-animal veterinary practice. Although not generally approved as an antidepressant or anti-anxiety medication, chlorphenamine appears to have these properties as well.[2][3]
Medical uses
Combination products
Chlorphenamine is often combined with phenylpropanolamine to form an allergy medication with both antihistamine and decongestant properties, though phenylpropanolamine is no longer available in the US after studies showed it increased the risk of stroke in young women. Chlorphenamine remains available with no such risk. Brand names had included Demazin, Allerest 12 Hour, Codral Nighttime, Chlornade, Contac 12 Hour, Exchange Select Allergy Multi-Symptom, A. R. M. Allergy Relief, Ordrine, Ornade Spansules, Teldrin, Triaminic, and Tylenol Cold/Allergy.
Chlorphenamine is combined with a narcotic (hydrocodone) in the product Tussionex, which is indicated for treatment of cough and upper respiratory symptoms associated with allergy or cold in adults and children 6 years of age and older.[4] This combination is manufactured as a time-released formula, which allows for administration every 12 hours, versus the more common 4-to-6-hour regimen for other narcotic cough suppressants.
Chlorphenamine/dihydrocodeine immediate-release syrups are also marketed. The antihistamine is helpful in cases where allergy or common cold is the reason for the cough; it is also a potentiator of opioids, allowing enhanced suppression of cough, analgesia, and other effects from a given quantity of the drug by itself. In various places in the world, cough & cold preparations containing codeine and chlorphenamine are available.
In the drug Coricidin, chlorphenamine is combined with the cough suppressant dextromethorphan.
Side effects
The adverse effects include drowsiness, dizziness, confusion, constipation, anxiety, nausea, blurred vision, restlessness, decreased coordination, dry mouth, shallow breathing, hallucinations, irritability, problems with memory or concentration, tinnitus and trouble urinating.
A large study linked the development of Alzheimer's disease and other forms of dementia to the use of chlorphenamine and other first-generation antihistamines, due to their anticholinergic properties.[5]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| SERT | 15.2 | Human | [7] |
| NET | 1,440 | Human | [7] |
| DAT | 1,060 | Human | [7] |
| 5-HT2A | 3,130 | Rat | [8] |
| 5-HT2C | 3,120 | Rat | [9] |
| H1 | 2.5–3.0 | Human | [10][11] |
| H2 | ND | ND | ND |
| H3 | >10,000 | Rat | [12] |
| H4 | 2,910 | Human | [13] |
| M1 | 25,700 | Human | [14] |
| M2 | 17,000 | Human | [14] |
| M3 | 52,500 | Human | [14] |
| M4 | 77,600 | Human | [14] |
| M5 | 28,200 | Human | [14] |
| hERG | 20,900 | Human | [15] |
| Values are Ki, unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. Values at the mAChRs and hERG are IC50 (nM). | |||
Chlorphenamine acts primarily as a potent H1antihistamine. It is specifically a potent inverse agonist of the histamine H1 receptor.[16][17] The drug is also commonly described as possessing weak anticholinergic activity by acting as an antagonist of the muscarinic acetylcholine receptors. The dextrorotatory stereoisomer, dexchlorpheniramine, has been reported to possess Kd values of 15 nM for the H1 receptor and 1,300 nM for the muscarinic acetylcholine receptors in human brain tissue.[18][19] The smaller the Kd value, the greater the binding affinity of the ligand for its target.
In addition to acting as an inverse agonist at the H1 receptor, chlorphenamine has been found to act as a potent serotonin reuptake inhibitor (Kd = 15.2 nM for the serotonin transporter).[7][20] It has only weak affinity for the norepinephrine and dopamine transporters (Kd = 1,440 nM and 1,060 nM, respectively).[7] A similar antihistamine, brompheniramine, led to the discovery of the selective serotonin reuptake inhibitor (SSRI) zimelidine. Limited clinical evidence shows that chlorphenamine is comparable to several antidepressant medications in its ability to inhibit the reuptake of serotonin and may be useful in the treatment of depression and anxiety disorders.[21][22]
A study found that dexchlorphenamine had Ki values for the human cloned H1 receptor of 2.67 to 4.81 nM while levchlorphenamine had Ki values of 211 to 361 nM for this receptor, indicating that dexchlorphenamine is the active enantiomer.[23] Another study found that dexchlorphenamine had a Ki value of 20 to 30 µM for the muscarinic acetylcholine receptor using rat brain tissue while levchlorphenamine had a Ki value of 40 to 50 µM for this receptor, indicating that both enantiomers have very low affinity for it.[24]
Pharmacokinetics
The elimination half-life of chlorphenamine has variously ranged between 13.9 and 43.4 hours in adults following a single dose in clinical studies.[1]
Chemistry
Chlorphenamine is an alkylamine and is a part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives including fluorpheniramine, dexchlorphenamine (Polaramine), brompheniramine (Dimetapp), dexbrompheniramine (Drixoral), deschlorpheniramine, and iodopheniramine. The halogenated alkylamine antihistamines all exhibit optical isomerism, and chlorphenamine in the indicated products is racemic chlorphenamine maleate, whereas dexchlorphenamine is the dextrorotary stereoisomer.
Synthesis
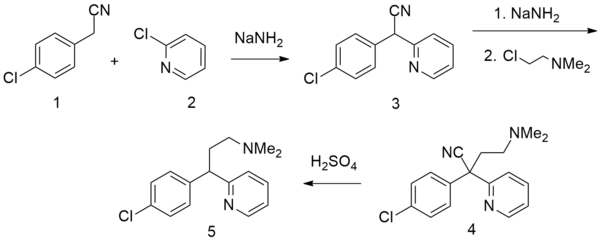
There are several patented methods for the synthesis of chlorphenamine. In one example, 4-chlorophenylacetonitrile is reacted with 2-chloropyridine in the presence of sodium amide to form 4-chlorophenyl(2-pyridyl)acetonitrile. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives γ-(4-chlorphenyl)-γ-cyano-N,N-dimethyl-2-pyridinepropanamine, the hydrolysis and decarboxylation of which lead to chlorphenamine.
A second method starts from pyridine, which undergoes alkylation by 4-chlorophenylacetonitrile,[25] giving 2-(4-chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives chlorphenamine.
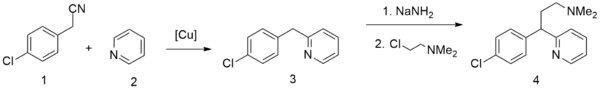
Society and culture
Generic names
Chlorphenamine is the INN while chlorpheniramine is the USAN and former BAN.
References
- 1 2 Yasuda SU, Wellstein A, Likhari P, Barbey JT, Woosley RL (1995). "Chlorpheniramine plasma concentration and histamine H1-receptor occupancy". Clin. Pharmacol. Ther. 58 (2): 210–20. doi:10.1016/0009-9236(95)90199-X. PMID 7648771.
- ↑ Carlsson, Arvid; Lindqvist, Margit. "Central and peripheral monoaminergic membrane-pump blockade by some addictive analgesics and antihistamines". Journal of Pharmacy and Pharmacology. Journal of Pharmacy and Pharmacology. Retrieved 1 December 2013.
- ↑ Gruetter CA, Lemke SM, Anestis DK, Szarek JL, Valentovic MA (Jul 1992). "Potentiation of 5-hydroxytryptamine-induced contraction in rat aorta by chlorpheniramine, citalopram and fluoxetine". Eur J Pharmacol. Department of Pharmacology, Marshall University School of Medicine, Huntington, WV. 217: 109–18. doi:10.1016/0014-2999(92)90827-q. PMID 1358631.
- ↑ "Tussionex® Pennkinetic® (hydrocodone polistirex and chlorpheniramine polistirex) Extended-Release Suspension" (PDF). UCB. 2011.
- ↑ Gray, Shelly L.; Anderson, Melissa L.; Dublin, Sascha; Hanlon, Joseph T.; Hubbard, Rebecca; Walker, Rod; Yu, Onchee; Crane, Paul K.; Larson, Eric B. (January 26, 2015). "Cumulative Use of Strong Anticholinergics and Incident Dementia: A Prospective Cohort Study". JAMA Intern. Med. 175 (3): 401–7. doi:10.1001/jamainternmed.2014.7663. PMC 4358759. PMID 25621434. Retrieved January 27, 2015.
- ↑ Roth, BL; Driscol, J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- 1 2 3 4 5 Tatsumi M, Groshan K, Blakely RD, Richelson E (1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". Eur. J. Pharmacol. 340 (2–3): 249–58. doi:10.1016/s0014-2999(97)01393-9. PMID 9537821.
- ↑ Hoffman BJ, Scheffel U, Lever JR, Karpa MD, Hartig PR (1987). "N1-methyl-2-125I-lysergic acid diethylamide, a preferred ligand for in vitro and in vivo characterization of serotonin receptors". J. Neurochem. 48 (1): 115–24. doi:10.1111/j.1471-4159.1987.tb13135.x. PMID 3794694.
- ↑ Sanders-Bush E, Breeding M (1988). "Putative selective 5-HT-2 antagonists block serotonin 5-HT-1c receptors in the choroid plexus". J. Pharmacol. Exp. Ther. 247 (1): 169–73. PMID 3139864.
- ↑ Moguilevsky N, Varsalona F, Noyer M, Gillard M, Guillaume JP, Garcia L, Szpirer C, Szpirer J, Bollen A (1994). "Stable expression of human H1-histamine-receptor cDNA in Chinese hamster ovary cells. Pharmacological characterisation of the protein, tissue distribution of messenger RNA and chromosomal localisation of the gene". Eur. J. Biochem. 224 (2): 489–95. doi:10.1111/j.1432-1033.1994.00489.x. PMID 7925364.
- ↑ Arias-Montaño JA, Young JM (1993). "Characteristics of histamine H1 receptors on HeLa cells". Eur. J. Pharmacol. 245 (3): 291–5. doi:10.1016/0922-4106(93)90110-u. PMID 8335064.
- ↑ West RE, Zweig A, Granzow RT, Siegel MI, Egan RW (1990). "Biexponential kinetics of (R)-alpha-[3H]methylhistamine binding to the rat brain H3 histamine receptor". J. Neurochem. 55 (5): 1612–6. doi:10.1111/j.1471-4159.1990.tb04946.x. PMID 2213013.
- ↑ Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, Rauser L, Lee SP, Lynch KR, Roth BL, O'Dowd BF (2001). "Discovery of a novel member of the histamine receptor family". Mol. Pharmacol. 59 (3): 427–33. PMID 11179435.
- 1 2 3 4 5 Yasuda SU, Yasuda RP (1999). "Affinities of brompheniramine, chlorpheniramine, and terfenadine at the five human muscarinic cholinergic receptor subtypes". Pharmacotherapy. 19 (4): 447–51. doi:10.1592/phco.19.6.447.31041. PMID 10212017.
- ↑ Suessbrich H, Waldegger S, Lang F, Busch AE (1996). "Blockade of HERG channels expressed in Xenopus oocytes by the histamine receptor antagonists terfenadine and astemizole". FEBS Lett. 385 (1–2): 77–80. doi:10.1016/0014-5793(96)00355-9. PMID 8641472.
- ↑ Simons, F. E. (November 18, 2004). "Advances in H1-Antihistamines". N Engl J Med. 351. (21): 2203–17. doi:10.1056/NEJMra033121. PMID 15548781.
- ↑ Leurs, R.; Church, M. K.; Taglialatela, M. (April 2002). "H1-antihistamines: inverse agonism, anti-inflammatory actions and cardiac effects". Clinical & Experimental Allergy. 32 (4): 483–651. doi:10.1046/j.0954-7894.2002.01314.x.
- ↑ Richelson E, Nelson A (1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. PMID 6086881.
- ↑ Cusack B, Nelson A, Richelson E (1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology. 114 (4): 559–65. doi:10.1007/bf02244985. PMID 7855217.
- ↑ Carlsson A, Lindqvist M (1969). "Central and peripheral monoaminergic membrane-pump blockade by some addictive analgesics and antihistamines". J. Pharm. Pharmacol. 21 (7): 460–4. doi:10.1111/j.2042-7158.1969.tb08287.x. PMID 4390069.
- ↑ Hellbom, E. (2006). "Chlorpheniramine, selective serotonin-reuptake inhibitors (SSRIs) and over-the-counter (OTC) treatment". Medical Hypotheses. 66 (4): 689–690. doi:10.1016/j.mehy.2005.12.006. PMID 16413139.
- ↑ Miyata S, Hirano S, Ohsawa M, Kamei J (2011). "Chlorpheniramine exerts anxiolytic-like effects and activates prefrontal 5-HT systems in mice". Psychopharmacology. 213 (2–3): 441–52. doi:10.1007/s00213-009-1695-0. PMID 19823805.
- ↑ Booth RG, Moniri NH, Bakker RA, Choksi NY, Nix WB, Timmerman H, Leurs R (2002). "A novel phenylaminotetralin radioligand reveals a subpopulation of histamine H(1) receptors". J. Pharmacol. Exp. Ther. 302 (1): 328–36. doi:10.1124/jpet.302.1.328. PMID 12065734.
- ↑ Yamamura HI, Snyder SH (1974). "Muscarinic cholinergic binding in rat brain". Proc. Natl. Acad. Sci. U.S.A. 71 (5): 1725–9. doi:10.1073/pnas.71.5.1725. PMC 388311. PMID 4151898.
- ↑ Djerassi, Carl (1948). "Brominations with Pyridine Hydrobromide Perbromide". Journal of the American Chemical Society. 70 (1): 417–418. doi:10.1021/ja01181a508.