Potassium channel blocker
Potassium channel blockers are agents which interfere with conduction through potassium channels.
Arrhythmia
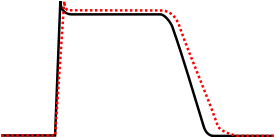
Potassium channel blockers used in the treatment of cardiac arrhythmia are classified as class III antiarrhythmic agents.
Mechanism
Class III agents predominantly block the potassium channels, thereby prolonging repolarization.[1] More specifically, their primary effect is on IKr.[2]
Since these agents do not affect the sodium channel, conduction velocity is not decreased. The prolongation of the action potential duration and refractory period, combined with the maintenance of normal conduction velocity, prevent re-entrant arrhythmias. (The re-entrant rhythm is less likely to interact with tissue that has become refractory).
Class III antiarrhythmic agents exhibit reverse use-dependent prolongation of the action potential duration (Reverse use-dependence). This means that the refractoriness of the ventricular myocyte increases at lower heart rates. This increases the susceptibility of the myocardium to Early Afterdepolarizations (EADs) at low heart rates. Antiarrhythmic agents that exhibit reverse use-dependence are more efficacious at preventing a tachyarrhythmia than converting someone into normal sinus rhythm. Because of the reverse use-dependence of class III agents, at low heart rates class III antiarrhythmic agents may paradoxically be more arrhythmogenic.
Examples and uses
- Amiodarone is indicated for the treatment of refractory VT or VF, particularly in the setting of acute ischemia. Amiodarone is also safe to use in individuals with cardiomyopathy and atrial fibrillation, to maintain normal sinus rhythm. Amiodarone prolongation of the action potential is uniform over a wide range of heart rates, so this drug does not have reverse use-dependent action. Amiodarone was the first agent described in this class.[3] Amiodarone should only be used to treat adults with life-threatening ventricular arrhythmias when other treatments are ineffective or have not been tolerated.[4]
- Dofetilide blocks only the rapid K channels; this means that at higher heart rates, when there is increased involvement of the slow K channels, dofetilide has less of an action potential-prolonging effect.
- Sotalol is indicated for the treatment of atrial or ventricular tachyarrhythmias, and AV re-entrant arrhythmias.
- Ibutilide is the only antiarrhythmic agent currently approved by the Food and Drug Administration for acute conversion of atrial fibrillation to sinus rhythm.
- Azimilide
- Bretylium
- Clofilium
- E-4031
- Nifekalant[5]
- Tedisamil
- Sematilide
Side effects
These agents include a risk of torsades de pointes.[6]
Anti-diabetics
Sulfonylureas come under the class of ATP-sensitive potassium channel blockers.
Other uses
Dalfampridine, A potassium channel blocker has also been approved for use in the treatment of multiple sclerosis.[7]
See also
References
- ↑ Lenz TL, Hilleman DE (July 2000). "Dofetilide, a new class III antiarrhythmic agent". Pharmacotherapy. 20 (7): 776–86. doi:10.1592/phco.20.9.776.35208. PMID 10907968.
- ↑ Riera AR, Uchida AH, Ferreira C, et al. (2008). "Relationship among amiodarone, new class III antiarrhythmics, miscellaneous agents and acquired long QT syndrome". Cardiol J. 15 (3): 209–19. PMID 18651412.
- ↑ "Milestones in the Evolution of the Study of Arrhythmias".
- ↑ "FDA MedWatch".
- ↑ Sahara M, Sagara K, Yamashita T, Iinuma H, Fu LT, Watanabe H (August 2003). "Nifekalant hydrochloride, a novel class III antiarrhythmic agent, suppressed postoperative recurrent ventricular tachycardia in a patient undergoing coronary artery bypass grafting and the Dor approach". Circ. J. 67 (8): 712–4. doi:10.1253/circj.67.712. PMID 12890916.
- ↑ "Introduction: Arrhythmias and Conduction Disorders: Merck Manual Professional".
- ↑ Judge SI, Bever CT (July 2006). "Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment". Pharmacol. Ther. 111 (1): 224–59. doi:10.1016/j.pharmthera.2005.10.006. PMID 16472864.