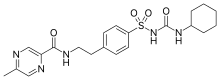
Glipizide
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Glucotrol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684060 |
| Pregnancy category | |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability |
100% (regular formulation) 90% (extended release) |
| Protein binding | 98 to 99% |
| Metabolism | Hepatic hydroxylation |
| Elimination half-life | 2 to 5 hours |
| Excretion | Renal and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard |
100.044.919 |
| Chemical and physical data | |
| Formula | C21H27N5O4S |
| Molar mass | 445.536 g/mol |
| 3D model (JSmol) | |
| Melting point | 208 to 209 °C (406 to 408 °F) |
| |
| |
| | |
Glipizide is an oral rapid- and short-acting anti-diabetic medication from the sulfonylurea class. It is classified as a second-generation sulfonylurea, which means that it undergoes enterohepatic circulation. Second-generation sulfonylureas are both more potent and have shorter half-lives than the first-generation sulfonylureas.
Originally available in 1984, it is marketed by Pfizer under the brand name Glucotrol in the USA, where Pfizer sells Glucotrol in doses of 5 and 10 milligrams and Glucotrol XL (an extended release form of glipizide) in doses of 2.5, 5, and 10 milligrams. Other companies also market glipizide, most commonly extended release tablets of 5 and 10 milligrams.
Mechanism of action
Glipizide sensitizes the beta cells of pancreatic islets of Langerhans insulin response, meaning that more insulin is released in response to glucose than would be without glipizide ingestion[1]. Glipizide acts by partially blocking potassium channels among beta cells of pancreatic islets of Langerhans. By blocking potassium channels, the cell depolarizes, which results in the opening of voltage-gated calcium channels. The resulting calcium influx encourages insulin release from beta cells. [2]
See also
References
- ↑ Drugs@FDA (the official database of FDA-approved drugs) https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020329s025lbl.pdf
- ↑ LH Bösenberg & DG van Zyl (2008) The mechanism of action of oral antidiabetic drugs: A review of recent literature, Journal of Endocrinology, Metabolism and Diabetes of South Africa, 13:3, 80-88, DOI: 10.1080/22201009.2008.1087217
External links
- Glucotrol XL Full U.S. Prescribing Information. Accessed on July 26, 2005.