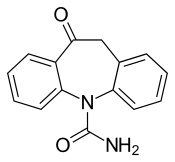
Oxcarbazepine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ɒks.kɑːrˈbæz.ɪˌpiːn/ |
| Trade names | Trileptal |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601245 |
| Pregnancy category |
|
| Routes of administration | By mouth (tablets or liquid)[1] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >95% |
| Metabolism | Liver (cytosolic enzymes and glucuronic acid) |
| Elimination half-life | 1–5 hours (healthy adults) |
| Excretion | Kidney (<1%)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard |
100.044.702 |
| Chemical and physical data | |
| Formula | C15H12N2O2 |
| Molar mass | 252.268 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Oxcarbazepine is an anticonvulsant drug primarily used in the treatment of epilepsy.[2] There is some evidence for oxcarbazepine as a mood-stabilizing agent and thus, it can be used as add-on therapy for bipolar disorder in patients that have failed or are unable to tolerate approved treatments.[3][4]
Common side effects include nausea, vomiting, dizziness, drowsiness, headache, double vision and trouble with walking.[2] Although not common, anaphylaxis may occur.[2] Due to its structural similarities to carbamazepine there is approximately a 25–30% chance of cross-reactivity between the two medications.[2]
Oxcarbazepine is marketed as Trileptal by Novartis and available in some countries as a generic drug.[5] There is also an extended-release formulation marketed as Oxtellar XR by Supernus Pharmaceuticals.[6]
Medical uses
Oxcarbazepine is an anticonvulsant used to reduce the occurrence of epileptic episodes, and is not intended to cure epilepsy.[7] Oxcarbazepine is used alone or in combination with other medications for the treatment of focal (partial) seizures in adults.[2] In pediatric populations, it can be used by itself for the treatment of partial seizures for children 4 years and older, or in combination with other medications for children 2 years and older.[2]
In addition, oxcarbazepine has been shown to improve mood and reduce anxiety; therefore, it is a potential option for add-on therapy in the treatment of bipolar disorder.[3][4]
Oxcarbazepine may also be beneficial for some types of neuropathic pain[8][9] and trigeminal neuralgia[10].
Pregnancy
Oxcarbazepine is listed as Pregnancy Category C.[2]
Currently, there is limited data analyzing the impact of oxcarbazepine on a human fetus.[2] Animal studies have shown an increased incidence of fetal abnormalities in pregnant rats and rabbits treated with oxcarbazepine, during pregnancy.[2] In addition, oxcarbazepine is structurally similar to carbamazepine (pregnancy category: D) which is considered to be teratogenic in humans.[2][11] Oxcarbazepine should only be used in pregnancy if the benefits justify the risks.[2]
Pregnant persons on oxcarbazepine should be closely monitored, as plasma levels of the active metabolite (MHD) has been shown to potentially decrease during pregnancy.[2]
Nursing mothers
Oxcarbazepine and MHD are both present in human breast milk and thus, some of the active drug can be transferred to a nursing infant.[2] When considering whether to continue this medication in nursing mothers, the impact of the drug's side effect profile on the infant, should be weighed against its anti-epileptic benefit for the mother.[2]
Adverse effects
Adverse effects are dose dependent. The most common include dizziness, blurred or double vision, nystagmus, ataxia, fatigue, headaches, nausea, vomiting, sleepiness, and difficulty in concentration and mental sluggishness.[2]
Other rare side effects of oxcarbazepine include severe low blood sodium (hyponatremia), anaphylaxis / angioedema, hypersensitivity (especially if experienced with carbamazepine, toxic epidermal necrolysis, Stevens–Johnson syndrome, anorgasmia [12]and thoughts of suicide.[2]
Measurement of serum sodium levels should be considered in maintenance treatment or if symptoms of hyponatremia develop.[2] Low blood sodium is seen in 20-30% of people taking oxcarbazepine and 8-12% of those who have oxcarbazpine-induced hyponatremia experience severe hyponatremia. Some side effects (such as headache) are more pronounced shortly after a dose is taken and tend to fade with the passage of time (60 to 90 minutes). Other side effects include stomach pain, tremor, rash, diarrhea, constipation, decreased appetite and dry mouth. Photosensitivity is a potential side-effect and people could experience severe sunburns as a result of sun exposure.[2]
Interactions
Oxcarbazepine and its active metabolite (monohydroxy derivative; MHD) have been shown to exhibit drug interactions when indicated with medications that are metabolized via CYP2C19, CYP3A4 and CYP3A5.[2] Oxcarbazepine and MHD is a potent inhibitor of CYP2C19 and thus, has the potential to increase plasma concentration of drugs that are metabolized through this pathway placing the patient at higher risk for toxicities.[2] Example of CYP2C19 substrates include: phenytoin, citalopram, diazepam, imipramine, omeprazole, propranolol, amitriptyline and progesterone when administered together.[2]
In addition, oxcarbazepine and MHD are CYP3A4 and CYP3A5 inducers and thus, have the potential to decrease the plasma concentration and therapeutic efficacy of medications that are CYP3A substrates.[2] Examples of CYP3A4 substrates include dihydropyridine calcium channel antagonist and oral contraceptives.[2] Due to the increase in drug metabolism of CYP3A4 substrates, hormonal contraceptives might be less effective, placing females at a greater risk of pregnancy.[2][7]
Furthermore, oxcarbazepine has been shown to interact with other anticonvulsant medications when used in combination.[2] For example, phenytoin and phenobarbital are known CYP inducers and thus, have the potential to decrease the plasma concentration of oxcarbazepine's active metabolite, MHD.[2]
Pharmacology
Oxcarbazepine and MHD exert their action by blocking voltage-sensitive sodium channels, thus leading to the stabilization of hyper excited neural membranes, suppression of repetitive neuronal firing and diminishment propagation of synaptic impulses.[2] Furthermore, anticonvulsant effects of these compounds could be attributed to enhanced potassium conductance and modulation of high-voltage activated calcium channels.[2]
Pharmacokinetics
Oxcarbazepine has high bioavailability once it is administrated orally as tablets.[2] Upon absorption, oxcarbazepine is largely metabolized to its pharmacologically active 10-monohydroxy metabolite licarbazepine (sometimes called MHD).[2] During a study in humans, the unchanged oxcarbazepine was found to be about 2% of the total radioactivity, while licarbazepine presented 70%, and the rest representing the minor metabolites.[2] The half-life of oxcarbazepine is considered to be about 2 hours, whereas licarbazepine has a half-life of nine hours. Therefore, the antiepileptic activity can be attributed to licarbazepine.[2]
Pharmacodynamics
Both oxcarbazepine and its MHD the active metabolite were found to show anticonvulsant properties in seizure models done on animals.[2] These compounds had protective functions whenever tonic extension seizures were induced electrically, but such protection was less apparent whenever seizures were induced chemically.[2] There was no observable tolerance during a four weeks course of treatment with daily administration of oxcarbazepine or MHD in electroshock test on mice and rats.[2]
Pharmacogenetics
Since the structure of oxcarbazepine is similar to carbamazepine, there is a consideration for genetic testing in patients who are of Asian descent (mainly populations of Han Chinese (2–12%), Thai (8%), Philippines (15%), Malaysia) due to the higher frequency of the HLA-B*1502 allele.[2] Human leukocyte antigen (HLA) allele B*1502 has been associated with an increased incidence of Stevens–Johnson syndrome and toxic epidermal necrolysis.[2]
Structure
Oxcarbazepine is a structural derivative of carbamazepine, with a ketone in place of the carbon–carbon double bond on the dibenzazepine ring at the 10 position (10-keto). This difference helps reduce the impact on the liver of metabolizing the drug, and also prevents the serious forms of anemia or agranulocytosis occasionally associated with carbamazepine. Aside from this reduction in side effects, it is thought to have the same mechanism as carbamazepine — sodium channel inhibition (presumed to be the main mechanism of action) – and is generally used to treat the same conditions.
Oxcarbazepine is a prodrug which is activated to licarbazepine in the liver.[13]
History
First made in 1965, it was patent-protected by Geigy in 1969 through DE 2011087. It was approved for use as an anticonvulsant in Denmark in 1990, Spain in 1993, Portugal in 1997, and eventually for all other EU countries in 1999. It was approved in the US in 2000.[5] In September 2010, Novartis pleaded guilty to marketing Trileptal for the unapproved uses of neuropathic pain and bipolar disorder.[14]
See also
References
- 1 2 "Oxcarbazepine Drug Label".
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 "DailyMed - OXCARBAZEPINE- oxcarbazepine tablet". dailymed.nlm.nih.gov. Retrieved 2015-11-11.
- 1 2 Mazza M, Di Nicola M, Martinotti G, Taranto C, Pozzi G, Conte G, Janiri L, Bria P, Mazza S (April 2007). "Oxcarbazepine in bipolar disorder: a critical review of the literature". Expert Opinion on Pharmacotherapy. 8 (5): 649–56. doi:10.1517/14656566.8.5.649. PMID 17376019.
- 1 2 Ghaemi SN, Berv DA, Klugman J, Rosenquist KJ, Hsu DJ (August 2003). "Oxcarbazepine treatment of bipolar disorder". The Journal of Clinical Psychiatry. 64 (8): 943–5. doi:10.4088/JCP.v64n0813. PMID 12927010.
- 1 2 "Trileptal® (oxcarbazepine) tablets and oral suspension". www.pharma.us.novartis.com. Retrieved 2015-11-11.
- ↑ "Neurology Portfolio | Supernus Pharmaceuticals". www.supernus.com. Retrieved 2015-11-11.
- 1 2 Information, National Center for Biotechnology; Pike, U. S. National Library of Medicine 8600 Rockville; MD, Bethesda; Usa, 20894. "Oxcarbazepine (By mouth) - National Library of Medicine - PubMed Health". mmdn/DNX1023. Retrieved 2015-11-02.
- ↑ Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, Jensen TS, Sindrup SH (November 2014). "The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study". Pain. 155 (11): 2263–73. doi:10.1016/j.pain.2014.08.014. PMID 25139589.
- ↑ Patel R, Kucharczyk M, Montagut-Bordas C, Lockwood S, Dickenson AH (August 2018). "Neuropathy following spinal nerve injury shares features with the irritable nociceptor phenotype: A back-translational study of oxcarbazepine". European Journal of Pain. doi:10.1002/ejp.1300. PMID 30091265.
- ↑ Cruccu G, Gronseth G, Alksne J, Argoff C, Brainin M, Burchiel K, Nurmikko T, Zakrzewska JM (October 2008). "AAN-EFNS guidelines on trigeminal neuralgia management". European Journal of Neurology. 15 (10): 1013–28. doi:10.1111/j.1468-1331.2008.02185.x. PMID 18721143.
- ↑ "DailyMed - CARBAMAZEPINE- carbamazepine tablet CARBAMAZEPINE- carbamazepine tablet, chewable". dailymed.nlm.nih.gov. Retrieved 2015-11-12.
- ↑ Boora K, Chiappone K, Dubovsky SL (2009). "Oxcarbazepine-induced reversible anorgasmia and ejaculatory failure: a case report". Primary Care Companion to the Journal of Clinical Psychiatry. 11 (4): 173–4. doi:10.4088/PCC.08l00688. PMC 2736039. PMID 19750073.
- ↑ Dulsat C, Mealy N, Castaner R, Bolos J (2009). "Eslicarbazepine acetate". Drugs of the Future. 34 (3): 189. doi:10.1358/dof.2009.034.03.1352675.
- ↑ "Novartis Pharmaceuticals Corp. to Pay More Than $420 Million to Resolve Off-label Promotion and Kickback Allegations | OPA | Department of Justice". www.justice.gov. Retrieved 2015-11-11.