Liafensine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| Chemical and physical data | |
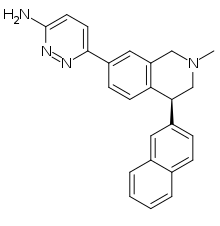
| Formula | C24H22N4 |
| Molar mass | 366.458 g/mol |
| 3D model (JSmol) | |
| |
| |
Liafensine (BMS-820836) is a serotonin-norepinephrine-dopamine reuptake inhibitor (SNDRI) which was under development by Bristol-Myers Squibb for the treatment of major depressive disorder.[1] Though it demonstrated comparable effectiveness to escitalopram and duloxetine in phase II clinical trials, development was terminated in 2013 because liafensine failed to show superior effectiveness relative to these drugs, a decision that was made likely based on its increased capacity for side effects as well as potential for abuse.[1]
See also
References
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.