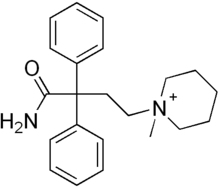
Fenpiverinium
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C22H29N2O+ |
| Molar mass | 337.48 g/mol |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Fenpiverinium is an anticholinergic and antispasmodic compound;[1] it is marketed as a combination drug with pitofenone hydrochloride and either nimesulide or metamizole in Eastern Europe and India to treat smooth muscle spasms and pain.[2]
The combination with metamizole was removed from the market in Lithuania for safety reasons in 2000[3] and a boxed warning against use by children and adolescents was added in Serbia in 2005.[4] In 2016 India banned marketing of the combination with nimesulide along with 344 other combination drugs;[5][6] the order was overturned in December and appealed by the Government in January 2017.[7]
References
- ↑ "Proposed INNs List 26" (PDF). WHO Chronicle. 25 (9). 1971. . "Recommended INNs List 12" (PDF). WHO Chronicle. 26 (10). 1973.
- ↑ Vardanyan, Ruben (2017). Piperidine-Based Drug Discovery. Elsevier. ISBN 9780128134283.
- ↑ Consolidated List of Products - Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or Not Approved by Governments, Twelfth Issue. WHO. 2005. p. 305.
- ↑ Consolidated List of Products Whose Consumption and/or Sale Have Been Banned, Withdrawn, Severely Restricted or not Approved by Governments, Fourteenth Issue. WHO. 2009. p. 35.
- ↑ "Health ministry bans 344 fixed dose combination drugs - Times of India". The Times of India. March 14, 2016.
- ↑ "Gazette of India D. L.-33004/99. PART II—Section 3—Sub-section (ii)" (PDF). Government of India. March 10, 2016.
- ↑ "Govt moves SC to enforce ban on 344 Fixed Dose Combination drugs". The Times of India. January 31, 2017.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.