| mAChRs | | Agonists | |
|---|
| Antagonists |
- 3-Quinuclidinyl benzilate
- 4-DAMP
- Aclidinium bromide (+formoterol)
- Abediterol
- AF-DX 250
- AF-DX 384
- Ambutonium bromide
- Anisodamine
- Anisodine
- Antihistamines (first-generation) (e.g., brompheniramine, buclizine, captodiame, chlorphenamine (chlorpheniramine), cinnarizine, clemastine, cyproheptadine, dimenhydrinate, dimetindene, diphenhydramine, doxylamine, meclizine, mepyramine (pyrilamine), mequitazine, perlapine, phenindamine, pheniramine, phenyltoloxamine, promethazine, propiomazine, triprolidine)
- AQ-RA 741
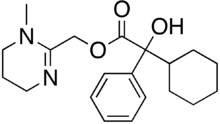
- Atropine
- Atropine methonitrate
- Atypical antipsychotics (e.g., clozapine, fluperlapine, olanzapine (+fluoxetine), rilapine, quetiapine, tenilapine, zotepine)
- Benactyzine
- Benzatropine (benztropine)
- Benzilone
- Benzilylcholine mustard
- Benzydamine
- BIBN 99
- Biperiden
- Bornaprine
- Camylofin
- CAR-226,086
- CAR-301,060
- CAR-302,196
- CAR-302,282
- CAR-302,368
- CAR-302,537
- CAR-302,668
- Caramiphen
- Cimetropium bromide
- Clidinium bromide
- Cloperastine
- CS-27349
- Cyclobenzaprine
- Cyclopentolate
- Darifenacin
- DAU-5884
- Desfesoterodine
- Dexetimide
- DIBD
- Dicycloverine (dicyclomine)
- Dihexyverine
- Difemerine
- Diphemanil metilsulfate
- Ditran
- EA-3167
- EA-3443
- EA-3580
- EA-3834
- Emepronium bromide
- Etanautine
- Etybenzatropine (ethybenztropine)
- Fenpiverinium
- Fentonium bromide
- Fesoterodine
- Flavoxate
- Glycopyrronium bromide (+beclometasone/formoterol, +indacaterol)
- Hexahydrodifenidol
- Hexahydrosiladifenidol
- Hexbutinol
- Hexocyclium
- Himbacine
- HL-031,120
- Homatropine
- Imidafenacin
- Ipratropium bromide (+salbutamol)
- Isopropamide
- J-104,129
- Hyoscyamine
- Mamba toxin 3
- Mamba toxin 7
- Mazaticol
- Mebeverine
- Meladrazine
- Mepenzolate
- Methantheline
- Methoctramine
- Methylatropine
- Methylhomatropine
- Methylscopolamine
- Metixene
- Muscarinic toxin 7
- N-Ethyl-3-piperidyl benzilate
- N-Methyl-3-piperidyl benzilate
- Nefopam
- Octatropine methylbromide (anisotropine methylbromide)
- Orphenadrine
- Otenzepad (AF-DX 116)
- Otilonium bromide
- Oxapium iodide
- Oxitropium bromide
- Oxybutynin
- Oxyphencyclimine
- Oxyphenonium bromide
- PBID
- PD-102,807
- PD-0298029
- Penthienate
- Pethidine
- pFHHSiD
- Phenglutarimide
- Phenyltoloxamine
- Pipenzolate bromide
- Piperidolate
- Pirenzepine
- Piroheptine
- Pizotifen
- Poldine
- Pridinol
- Prifinium bromide
- Procyclidine
- Profenamine (ethopropazine)
- Propantheline bromide
- Propiverine
- Quinidine
- Revefenacin
- Rociverine
- RU-47,213
- SCH-57,790
- SCH-72,788
- SCH-217,443
- Scopolamine (hyoscine)
- Scopolamine butylbromide (hyoscine butylbromide)
- Silahexacyclium
- Sofpironium bromide
- Solifenacin
- SSRIs (e.g., femoxetine, paroxetine)
- Telenzepine
- Terodiline
- Tetracyclic antidepressants (e.g., amoxapine, maprotiline, mianserin, mirtazapine)
- Tiemonium iodide
- Timepidium bromide
- Tiotropium bromide
- Tiquizium bromide
- Tofenacin
- Tolterodine
- Tricyclic antidepressants (e.g., amitriptyline (+perphenazine), amitriptylinoxide, butriptyline, cidoxepin, clomipramine, desipramine, desmethyldesipramine, dibenzepin, dosulepin (dothiepin), doxepin, imipramine, lofepramine, nitroxazepine, northiaden (desmethyldosulepin), nortriptyline, protriptyline, quinupramine, trimipramine)
- Tridihexethyl
- Trihexyphenidyl
- Trimebutine
- Tripitamine (tripitramine)
- Tropacine
- Tropatepine
- Tropicamide
- Trospium chloride
- Typical antipsychotics (e.g., chlorpromazine, chlorprothixene, cyamemazine (cyamepromazine), loxapine, mesoridazine, thioridazine)
- Umeclidinium bromide (+vilanterol)
- WIN-2299
- Xanomeline
- Zamifenacin
|
|---|
|
|---|