Transient receptor potential channel
| Transient receptor potential (TRP) ion channel | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | TRP | ||||||||
| Pfam | PF06011 | ||||||||
| InterPro | IPR010308 | ||||||||
| OPM superfamily | 8 | ||||||||
| OPM protein | 3j5p | ||||||||
| Membranome | 605 | ||||||||
| |||||||||
Transient receptor potential channels (TRP channels) are a group of ion channels located mostly on the plasma membrane of numerous animal cell types. There are about 28 TRP channels that share some structural similarity to each other.[1] These are grouped into two broad groups: Group 1 includes TRPC ( "C" for canonical), TRPV ("V" for vanilloid), TRPM ("M" for melastatin), TRPN, and TRPA. In group 2, there are TRPP ("P" for polycystic) and TRPML ("ML" for mucolipin). Many of these channels mediate a variety of sensations like the sensations of pain, hotness, warmth or coldness, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and used in animals to sense hot or cold.[2] Some TRP channels are activated by molecules found in spices like garlic (allicin), chilli pepper (capsaicin), wasabi (allyl isothiocyanate); others are activated by menthol, camphor, peppermint, and cooling agents; yet others are activated by molecules found in cannabis (i.e., THC, CBD and CBN) or stevia. Some act as sensors of osmotic pressure, volume, stretch, and vibration.
These ion channels are relatively non-selectively permeable to cations, including sodium, calcium and magnesium. TRP channels were initially discovered in trp-mutant strain of the fruit fly Drosophila. Later, TRP channels were found in vertebrates where they are ubiquitously expressed in many cell types and tissues. Most TRP channels are composed of 6 membrane-spanning helices with intracellular N- and C-termini. Mammalian TRP channels are activated and regulated by a wide variety of stimuli and are expressed throughout the body.
Sub-families
They are encoded by at least 28–30[3] channel subunit genes divided into seven sub-families:
- Canonical (TRPC)
- Associated with focal segmental glomerulosclerosis.
- Vanilloid (TRPV)
- TRPV1 mediates the pungent odour and pain/hot sensations associated with capsaicin and piperine.
- Ankyrin (TRPA)
- Stress (mechanical) receptor. Disputed to be temperature-sensitive; activated by isothiocyanates (pungent chemicals in substances such as mustard oil and wasabi).
- Melastatin (TRPM)
- Associated with hypomagnesemia with secondary hypocalcemia.
- Polycystic (TRPP)
- Associated with polycystic kidney disease.
- Mucolipin (TRPML)
- Associated with mucolipidosis type IV.
- No mechanoreceptor potential C (NOMPC; TRPN)
- Not found in mammals.
Structure
Most TRP channels are composed of 6 membrane-spanning helices with intracellular N- and C-termini. Mammalian TRP channels are activated and regulated by a wide variety of stimuli including many post-transcriptional mechanisms like phosphorylation, G-protein receptor coupling, ligand-gating, and ubiquitination. The receptors are found in almost all cell types and largely localized in the cell membrane, modulating ion entry.
Function
TRP channels modulate ion entry driving forces and Ca2+ and Mg2+ transport machinery in the plasma membrane, where most of them are located. TRPs have important interactions with other proteins and often form signaling complexes, the exact pathways of which are unknown.[4] TRP channels were initially discovered in the trp mutant strain of the fruit fly Drosophila [5] which displayed transient elevation of potential in response to light stimuli and were so named transient receptor potential channels.[6] TRPML channels function as intracellular calcium release channels and thus serve an important role in organelle regulation.[4] Importantly, many of these channels mediate a variety of sensations like the sensations of pain, hotness, warmth or coldness, different kinds of tastes, pressure, and vision. In the body, some TRP channels are thought to behave like microscopic thermometers and are used in animals to sense hot or cold. TRPs act as sensors of osmotic pressure, volume, stretch, and vibration. TRPs have been seen to have complex multidimensional roles in sensory signaling. Many TRPs function as intracellular calcium release channels.
Pain and temperature sensation
TRP ion channels convert energy into action potentials in somatosensory nociceptors.[7] Thermo-TRP channels have a C-terminal domain that is responsible for thermosensation and have a specific interchangeable region that allows them to sense temperature stimuli that is tied to ligand regulatory processes.[8] Although most TRP channels are modulated by changes in temperature, some have a crucial role in temperature sensation. There are at least 6 different Thermo-TRP channels and each plays a different role. For instance, TRPM8 relates to mechanisms of sensing cold, TRPV1 and TRPM3 contribute to heat and inflammation sensations, and TRPA1 facilitates many signaling pathways like sensory transduction, nociception, inflammation and oxidative stress.[7]
Taste
TRPM5 is involved in taste signaling of sweet, bitter and umami tastes by modulating the signal pathway in taste receptor cells.[9] The TRP channels play a significant role in taste with channels responding to different tastes. TRPA1 responds to mustard oil (allyl isothiocyanate), wasabi, and cinnamon, TRPA1 and TRPV responds to garlic (allicin), TRPV1 responds to chilli pepper (capsaicin), TRPM8 is activated by menthol, camphor, peppermint, and cooling agents; TRPV2 is activated by molecules (THC, CBD and CBN) found in marijuana; TRPM5 is activated by the sweet glycosides found in the stevia plant.
TRP-like channels in insect vision
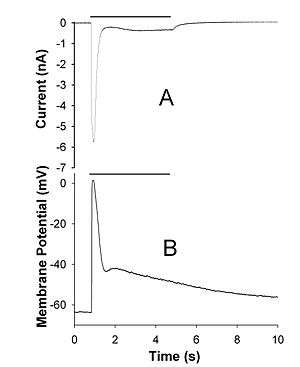
The trp-mutant fruit flies, which lack a functional copy of trp gene, are characterized by a transient response to light, unlike wild-type flies that demonstrate a sustained photoreceptor cell activity in response to light.[10] A distantly related isoform of TRP channel, TRP-like channel (TRPL), was later identified in Drosophila photoreceptors, where it is expressed at approximately 10- to 20-fold lower levels than TRP protein. A mutant fly, trpl, was subsequently isolated. Apart from structural differences, the TRP and TRPL channels differ in cation permeability and pharmacological properties.
TRP/TRPL channels are solely responsible for depolarization of insect photoreceptor plasma membrane in response to light. When these channels open, they allow sodium and calcium to enter the cell down the concentration gradient, which depolarizes the membrane. Variations in light intensity affect the total number of open TRP/TRPL channels, and, therefore, the degree of membrane depolarization. These graded voltage responses propagate to photoreceptor synapses with second-order retinal neurons and further to the brain.
It is important to note that the mechanism of insect photoreception is dramatically different from that in mammals. Excitation of rhodopsin in mammalian photoreceptors leads to the hyperpolarization of the receptor membrane but not to depolarization as in the insect eye. In Drosophila and, it is presumed, other insects, a phospholipase C (PLC)-mediated signaling cascade links photoexcitation of rhodopsin to the opening of the TRP/TRPL channels. Although numerous activators of these channels such as phosphatidylinositol-4,5-bisphosphate (PIP2) and polyunsaturated fatty acids (PUFAs) were known for years, a key factor mediating chemical coupling between PLC and TRP/TRPL channels remained a mystery until recently. It was found that breakdown of a lipid product of PLC cascade, diacylglycerol (DAG), by the enzyme Diacylglycerol lipase, generates PUFAs that can activate TRP channels, thus initiating membrane depolarization in response to light.[11] This mechanism of TRP channel activation may be well-preserved among other cell types where these channels perform various functions.
Clinical significance
Mutations in TRPs have been linked to neurodegenerative disorders, skeletal dysplasia, kidney disorders,[4] and may play an important role in cancer. TRPs may make important therapeutic targets. There is significant clinical significance to TRPV1, TRPV2, TRPV3 and TRPM8’s role as thermoreceptors, and TRPV4 and TRPA1’s role as mechanoreceptors; reduction of chronic pain may be possible by targeting ion channels involved in thermal, chemical, and mechanical sensation to reduce their sensitivity to stimuli.[12] For instance the use of TRPV1 agonists would potentially inhibit nociception at TRPV1, particularly in pancreatic tissue where TRPV1 is highly expressed.[13] The TRPV1 agonist capsaicin, found in chili peppers, has been indicated to relieve neuropathic pain.[4] TRPV1 agonists inhibit nociception at TRPV1
Role in cancer
Altered expression of TRP proteins often leads to tumorigenesis, clearly seen in TRPM1 and TRPV6.[13] Particularly high levels of TRPM1 and TRPV6 in prostate cancer and of TRPM1 in melanomas have been noted. Such observations could be helpful in following cancer progression and could lead to the development of drugs over activating ion channels, leading to apoptosis and necrosis. Much research remains to be done as to whether TRP channel mutations lead to cancer progression or whether they are associated mutations.
Role in inflammatory responses
In addition to TLR4 mediated pathways, certain members of the family of the transient receptor potential ion channels recognize LPS. LPS-mediated activation of TRPA1 was shown in mice[14] and Drosophila melanogaster flies.[15] At higher concentrations, LPS activates other members of the sensory TRP channel family as well, such as TRPV1, TRPM3 and to some extent TRPM8 [16]. LPS is recognized by TRPV4 on epithelial cells. TRPV4 activation by LPS was necessary and sufficient to induce nitric oxide production with a bactericidal effect.[17]
History of Drosophila TRP channels
The original TRP-mutant in Drosophila was first described by Cosens and Manning in 1969 as "a mutant strain of D. melanogaster which, though behaving phototactically positive in a T-maze under low ambient light, is visually impaired and behaves as though blind". It also showed an abnormal ERG response to light[10] and it was investigated subsequently by Baruch Minke, a post-doc in the group of William Pak, and named TRP according to its behavior in the ERG.[18] The identity of the mutated protein was unknown until it was cloned by Craig Montell, a post-doctoral researcher in Gerald Rubin's research group, in 1989, who noted its predicted structural relationship to channels known at the time [19] and Roger Hardie and Baruch Minke who provided evidence in 1992 that it is an ion channel that opens in response to light stimulation.[20] The TRPL channel was cloned and characterized in 1992 by the research group of Leonard Kelly.[21]
References
- ↑ Islam MS, ed. (January 2011). Transient Receptor Potential Channels. Advances in Experimental Medicine and Biology. 704. Berlin: Springer. p. 700. ISBN 978-94-007-0264-6.
- ↑ Vriens J, Nilius B, Voets T (September 2014). "Peripheral thermosensation in mammals". Nature Reviews. Neuroscience. 15 (9): 573–89. doi:10.1038/nrn3784. PMID 25053448.
- ↑ Zheng J. Molecular Mechanism of TRP Channels. Comprehensive Physiology. 2013;3(1):221-242. doi:10.1002/cphy.c120001, L.J. Wu, T.B. Sweet, D.E. Clapham International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family Pharmacol Rev, 62 (2010), pp. 381–404
- 1 2 3 4 Winston KR, Lutz W (March 1988). "Linear accelerator as a neurosurgical tool for stereotactic radiosurgery". Neurosurgery. 22 (3): 454–64. doi:10.1097/00006123-198803000-00002. PMID 3129667.
- ↑ Cosens DJ, Manning A (October 1969). "Abnormal electroretinogram from a Drosophila mutant". Nature. 224 (5216): 285–7. doi:10.1038/224285a0. PMID 5344615.
- ↑ Montell C, Rubin GM (April 1989). "Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction". Neuron. 2 (4): 1313–23. doi:10.1016/0896-6273(89)90069-x. PMID 2516726.
- 1 2 Eccles R (1989). "Nasal physiology and disease with reference to asthma". Agents and Actions. Supplements. 28: 249–61. PMID 2683630.
- ↑ Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R (May 2006). "A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels". The Journal of Neuroscience. 26 (18): 4835–40. doi:10.1523/JNEUROSCI.5080-05.2006. PMID 16672657.
- ↑ Philippaert K, Pironet A, Mesuere M, Sones W, Vermeiren L, Kerselaers S, Pinto S, Segal A, Antoine N, Gysemans C, Laureys J, Lemaire K, Gilon P, Cuypers E, Tytgat J, Mathieu C, Schuit F, Rorsman P, Talavera K, Voets T, Vennekens R (March 2017). "Steviol glycosides enhance pancreatic beta-cell function and taste sensation by potentiation of TRPM5 channel activity". Nature Communications. 8: 14733. doi:10.1038/ncomms14733. PMC 5380970. PMID 28361903.
- 1 2 Cosens DJ, Manning A (October 1969). "Abnormal electroretinogram from a Drosophila mutant". Nature. 224 (5216): 285–7. doi:10.1038/224285a0. PMID 5344615.
- ↑ Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, An L, Doerge RW, Pak WL (June 2008). "DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors". Neuron. 58 (6): 884–96. doi:10.1016/j.neuron.2008.05.001. PMC 2459341. PMID 18579079.
- ↑ Levine JD, Alessandri-Haber N (August 2007). "TRP channels: targets for the relief of pain". Biochimica et Biophysica Acta. 1772 (8): 989–1003. doi:10.1016/j.bbadis.2007.01.008. PMID 17321113.
- 1 2 Prevarskaya N, Zhang L, Barritt G (August 2007). "TRP channels in cancer". Biochimica et Biophysica Acta. 1772 (8): 937–46. doi:10.1016/j.bbadis.2007.05.006. PMID 17616360.
- ↑ Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, et al. (20 January 2014). "TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins". Nature Communications. 5: 3125. doi:10.1038/ncomms4125. PMID 24445575.
- ↑ Soldano A, Alpizar YA, Boonen B, Franco L, López-Requena A, Liu G, Mora N, Yaksi E, Voets T, Vennekens R, Hassan BA, Talavera K (June 2016). "Gustatory-mediated avoidance of bacterial lipopolysaccharides via TRPA1 activation in Drosophila". eLife. 5. doi:10.7554/eLife.13133. PMID 27296646.
- ↑ Boonen B, Alpizar YA, Sanchez A, López-Requena A, Voets T, Talavera K (April 2018). "Differential effects of lipopolysaccharide on mouse sensory TRP channels". Cell Calcium. 73: 72–81. doi:10.1016/j.ceca.2018.04.004. PMID 29689522.
- ↑ Alpizar YA, Boonen B, Sanchez A, Jung C, López-Requena A, Naert R, et al. (October 2017). "TRPV4 activation triggers protective responses to bacterial lipopolysaccharides in airway epithelial cells". Nature Communications. 8 (1): 1059. doi:10.1038/s41467-017-01201-3. PMID 29057902.
- ↑ Minke B, Wu C, Pak WL (November 1975). "Induction of photoreceptor voltage noise in the dark in Drosophila mutant". Nature. 258 (5530): 84–7. doi:10.1038/258084a0. PMID 810728.
- ↑ Montell C, Rubin GM (April 1989). "Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction". Neuron. 2 (4): 1313–23. doi:10.1016/0896-6273(89)90069-X. PMID 2516726.
- ↑ Hardie RC, Minke B (April 1992). "The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors". Neuron. 8 (4): 643–51. doi:10.1016/0896-6273(92)90086-S. PMID 1314617.
- ↑ Phillips AM, Bull A, Kelly LE (April 1992). "Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene". Neuron. 8 (4): 631–42. doi:10.1016/0896-6273(92)90085-R. PMID 1314616.
Further reading
External links
- Transient+Receptor+Potential+Channels at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Transient Receptor Potential Channels". IUPHAR Database of Receptors and Ion Channels. International Union of Basic and Clinical Pharmacology.
- Clapham DE, DeCaen P, Carvacho I, Chaudhuri D, Doerner JF, Julius D, Kahle KT, McKemy D, Oancea E, Sah R, Stotz SC, Tong D, Wu L, Xu H, Nilius B, Owsianik G. "Transient Receptor Potential channels". IUPHAR/BPS Guide to Pharmacology.
- "TRIP Database". a manually curated database of protein-protein interactions for mammalian TRP channels.