Triphenylphosphine oxide
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Triphenyl-λ5-phosphanone | |
| Other names
Triphenylphosphine oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.217 |
PubChem CID |
|
| RTECS number | SZ1676000 |
| |
| |
| Properties | |
| C18H15OP | |
| Molar mass | 278.29 g/mol |
| Appearance | white crystals |
| Density | 1.212g/cm^3 |
| Melting point | 154 to 158 °C (309 to 316 °F; 427 to 431 K) |
| Boiling point | 360 °C (680 °F; 633 K) |
| low | |
| Solubility in other solvents | polar organic solvents |
| Structure | |
| tetrahedral | |
| Hazards | |
| Main hazards | slight |
| R-phrases (outdated) | 22-36/37/38 |
| S-phrases (outdated) | 26 |
| Related compounds | |
Related compounds |
P(C6H5)3S; |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
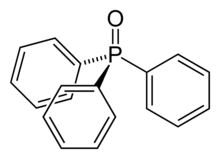
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallizing of chemical compounds.
Structure and properties
Ph3PO is tetrahedral molecule related to POCl3.[1] The oxygen center is relatively basic. The rigidity of the backbone and the basicity of the oxygen center make this species a popular agent to crystallize otherwise difficult to crystallize molecules. This trick is applicable to molecules that have acidic hydrogen atoms, e.g. phenols.[2]
Up to now, several modifications of Ph3PO have been found: For example, a monoclinic form crystalizes in the space group P21/c with Z = 4 and a = 15.066(1) Å, b = 9.037(2) Å, c = 11.296(3) Å, and β = 98.47(1)°.[3] The orthorhombic modification crystallizes in the space group Pbca with Z = 4 and 29.089(3) Å, b = 9.1347(9), c = 11.261(1) Å.[4]
As a by-product of organic synthesis
Ph3PO is a by-product of many useful reactions in organic synthesis including the Wittig, Staudinger, and Mitsunobu reactions. It is also formed when PPh3Cl2 is employed to convert alcohols into alkyl chlorides.
- Ph3PCl2 + ROH → Ph3PO + HCl + RCl
Triphenylphosphine can be regenerated from the oxide by treatment with trichlorosilane.
- Ph3PO + SiHCl3 → PPh3 + 1/n (OSiCl2)n + HCl
Triphenylphosphine oxide can be difficult to remove from reaction mixtures by means of chromatography. It is poorly soluble in hexane and cold diethyl ether. Trituration or chromatography of crude products with these solvents often leads to a good separation of triphenylphosphine oxide. Its removal is facilitated by conversion to its Mg(II) complex, which is poorly soluble in toluene or dichloromethane and can be filtered off.[5]
Coordination chemistry
nickel(II)-from-xtal-3D-balls.png)
Ph3PO is a ligand for "hard" metal centers. A representative complex is the tetrahedral species NiCl2(OPPh3)2.[6]
Ph3PO is a common impurity in PPh3. The oxidation of PPh3 by oxygen, including air, is catalyzed by many metal ions:
- 2 PPh3 + O2 → 2 Ph3PO
References
- ↑ D. E. C. Corbridge "Phosphorus: An Outline of its Chemistry, Biochemistry, and Technology" 5th Edition Elsevier: Amsterdam. ISBN 0-444-89307-5.
- ↑ M. C. Etter and P. W. Baures (1988). "Triphenylphosphine oxide as a crystallization aid". J. Am. Chem. Soc. 110 (2): 639–640. doi:10.1021/ja00210a076.
- ↑ Spek, Anthony L. (1987). "Structure of a second monoclinic polymorph of triphenylphosphine oxide". Acta Crystallographica. C43: 1233–1235. doi:10.1107/S0108270187092345.
- ↑ Al-Farhan, Khalid A. (1992). "Crystal structure of triphenylphosphine oxide". Journal of Crystallographic and Spectroscopic Research. 22 (6): 687–689. doi:10.1007/BF01160986.
- ↑ Patent WO 1998007724. "Process for the preparation of 7-alkoxyalkyl-1,2,4-triazolo[1,5-a] pyrimidine derivatives"
- ↑ D. M. L. Goodgame and M. Goodgame (1965). "Near-Infrared Spectra of Some Pseudotetrahedral Complexes of Cobalt (II) and Nickel(II)". Inorg. Chem. 4 (2): 139–143. doi:10.1021/ic50024a002.