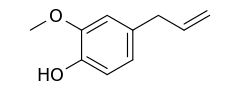
Eugenol
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methoxy-4-(prop-2-en-1-yl)phenol | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.355 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.20 g·mol−1 |
| Density | 1.06 g/cm3 |
| Melting point | −7.5 °C (18.5 °F; 265.6 K) |
| Boiling point | 254 °C (489 °F; 527 K) |
| Acidity (pKa) | 10.19 at 25 °C |
| −1.021×10−4 cm3/mol | |
| Hazards | |
| NFPA 704 | |
| Flash point | 104 °C (219 °F; 377 K) |
| Related compounds | |
Related compounds |
2-Phenethyl propionate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Eugenol /ˈjuːdʒɪnɒl/ is a phenylpropene and an allyl chain-substituted guaiacol. Eugenol is a member of the phenylpropanoids class of chemical compounds. It is a colorless to pale yellow, aromatic oily liquid extracted from certain essential oils especially from clove oil, nutmeg, cinnamon, basil and bay leaf.[1][2][3][4] It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil.[5] Eugenol has a pleasant, spicy, clove-like odor.[6] The name is derived from Eugenia caryophyllata, a former scientific name for cloves.
Modern uses
Eugenol is used in perfumes, flavorings, and essential oils. It is also used as a local antiseptic and anaesthetic.[7][8] Eugenol can be combined with zinc oxide to form zinc oxide eugenol which has restorative and prosthodontic applications in dentistry. For example, zinc oxide eugenol is used for root canal sealing.[9]
Attempts have been made to develop eugenol derivatives as intravenous anesthetics, as an alternative to propanidid which produces unacceptable side effects around the site of injection in many patients.[10]
It can be used to reduce the presence of Listeria monocytogenes and Lactobacillus sakei in food.[11]
It is also used in manufacturing stabilizers and antioxidants for plastics and rubbers.
It is one of many compounds that is attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.[12] It also attracts female cucumber beetle.[13] It was recently discovered that eugenol and isoeugenol, floral volatile scent compounds, are catalyzed by a single type of enzyme in the genus Gymnadenia and the gene encoding for this enzyme is the first functionally characterized gene in these species so far.[14]
Clove oil is growing in popularity as an anaesthetic for use on aquarium fish as well as on wild fish when sampled for research and management purposes.[15][16] Where readily available, it presents a humane method to euthanise sick and diseased fish either by direct overdose or to induce sleep before an overdose of eugenol.[17]
Biosynthesis
The biosynthesis of eugenol begins with the amino acid tyrosine. L-tyrosine is converted to p-coumaric acid by the enzyme tyrosine ammonia lyase (TAL).[18] From here, p-coumaric acid is converted to caffeic acid by p-coumarate 3-hydroxylase using oxygen and NADPH. S-Adenosyl methionine (SAM) is then used to methylate caffeic acid, forming ferulic acid, which is in turn converted to feruloyl-CoA by the enzyme 4-hydroxycinnamoyl-CoA ligase (4CL).[19] Next, feruloyl-CoA is reduced to coniferaldehyde by cinnamoyl-CoA reductase (CCR). Coniferaldeyhyde is then further reduced to coniferyl alcohol by cinnamyl-alcohol dehydrogenase (CAD) or sinapyl-alcohol dehydrogenase (SAD). Coniferyl alcohol is then converted to an ester in the presence of the substrate CH3COSCoA, forming coniferyl acetate. Finally, coniferyl acetate is converted to eugenol via the enzyme eugenol synthase 1 and the use of NADPH.

Toxicity
Eugenol is hepatotoxic, meaning it may cause damage to the liver.[20][21] Overdose is possible, causing a wide range of symptoms from blood in the patient's urine, to convulsions, diarrhoea, nausea, unconsciousness, dizziness, or rapid heartbeat.[22] According to a published 1993 report, a 2-year-old boy nearly died after taking between 5 and 10 ml.[23]
Allergy
Eugenol is subject to restrictions on its use in perfumery[24] as some people may become sensitised to it, however, the degree to which eugenol can cause an allergic reaction in humans is disputed.[25]
Eugenol is a component of balsam of Peru, to which some people are allergic.[26][27] When eugenol is used in dental preparations such as surgical pastes, dental packing, and dental cement, it may cause contact stomatitis and allergic cheilitis.[26] The allergy can be discovered via a patch test.[26]
Natural occurrence
Eugenol naturally occurs in several plants, including the following:
- Cloves (Syzygium aromaticum)[28][29][30]
- Wormwood
- Cinnamon[29][31]
- Cinnamomum tamala[32]
- Nutmeg (Myristica fragrans)[33]
- Ocimum basilicum (sweet basil)[34]
- Ocimum gratissimum (African basil)[14][35]
- Ocimum tenuiflorum (syn. Ocimum sanctum, tulsi or holy basil)
- Japanese star anise[36]
- Lemon balm[37]
- Dill
- Pimenta racemosa
- Vanilla
- Bay laurel
- Celery
- Ginger
See also
References
- ↑ "Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum L.) Alston" (PDF). Bangladesh Council of Scientific and Industrial Research BCSIR Laboratories. 4: 451–454.
- ↑ Mallavarapu, Gopal R.; Ramesh, S.; Chandrasekhara, R. S.; Rajeswara Rao, B. R.; Kaul, P. N.; Bhattacharya, A. K. (1995). "Investigation of the essential oil of cinnamon leaf grown at Bangalore and Hyderabad". Flavour and Fragrance Journal. 10 (4): 239–242. doi:10.1002/ffj.2730100403.
- ↑ Yield and Oil Composition of 38 Basil (Ocimum basilicum L.) Accessions Grown in Mississippi Archived 15 October 2010 at the Wayback Machine.
- ↑ "Typical G.C. for bay leaf oil". Thegoodscentscompany.com. Retrieved 2014-04-27.
- ↑ Barnes, J.; Anderson, L. A.; Phillipson, J. D. (2007) [1996]. Herbal Medicines (PDF) (3rd ed.). London: Pharmaceutical Press. ISBN 978-0-85369-623-0.
- ↑ "Human Metabolome Database: Showing metabocard for Eugenol (HMDB0005809)". www.hmdb.ca. Retrieved 2018-07-01.
- ↑ Sell, AB; Carlini, EA (1976). "Anesthetic action of methyleugenol and other eugenol derivatives". Pharmacology. 14 (4): 367–77. doi:10.1159/000136617. PMID 935250.
- ↑ Jadhav, B. K.; Khandelwal, K. R.; Ketkar, A. R.; Pisal, S. S. (February 2004). "Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases". Drug Development and Industrial Pharmacy. 30 (2): 195–203. doi:10.1081/DDC-120028715. PMID 15089054.
- ↑ Ferracane, Jack L. (2001). Materials in Dentistry: Principles and Applications (2nd ed.). Lippincott, Williams & Wilkins. ISBN 978-0-7817-2733-4.
- ↑ Right, D. A.; Payne, J. P. (June 1962). "A clinical study of intravenous anaesthesia with a eugenol derivative, G.29.505" (abstract). British Journal of Anaesthesia. 34 (6): 379–385. doi:10.1093/bja/34.6.379. PMID 14008420.
- ↑ Gill, A. O.; Holley, R. A. (2004). "Mechanisms of Bactericidal Action of Cinnamaldehyde against Listeria monocytogenes and of Eugenol against L. Monocytogenes and Lactobacillus sakei". Applied and Environmental Microbiology. 70 (10): 5750–5. doi:10.1128/AEM.70.10.5750-5755.2004. PMC 522076. PMID 15466510.
- ↑ Schiestl, F. P.; Roubik, D. W. (January 2003). "Odor Compound Detection in Male Euglossine Bees". Journal of Chemical Ecology. 29 (1): 253–257. doi:10.1023/A:1021932131526. PMID 12647866. Archived from the original on 3 February 2013. Retrieved 4 February 2009.
- ↑ "Cucumber Beetles: Organic and Biorational Integrated Pest Management (Summary)". Attra.ncat.org. 2013-08-05. Retrieved 2014-04-27.
- 1 2 Gupta, A. K.; Schauvinhold, I.; Pichersky, E.; Schiestl, F. P. (2014). "Eugenol synthase genes in floral scent variation in Gymnadenia species". Functional & Integrative Genomics. 14 (4): 779–788. doi:10.1007/s10142-014-0397-9. PMID 25239559.
- ↑ Anesthesia, Analgesia, and Surgery in Pet Fish. Atlantic Coast Veterinary Conference. 2001. Retrieved 2014-03-17.
- ↑ Grush, J.; Noakes, D. L. G.; Moccia, R. D. (February 2004). "The Efficacy of Clove Oil As An Anesthetic for the Zebrafish". Zebrafish. 1 (1): 46–53. doi:10.1089/154585404774101671. PMID 18248205.
- ↑ Monks, Neale (2009-04-02). "Aquarium Fish Euthanasia" (PDF). Fish Channel. Retrieved 2010-12-07.
- ↑ Dewick, P. M. (2009). Medicinal Natural Products. John Wiley & Sons. doi:10.1002/9780470742761. ISBN 9780470742761.
- ↑ Harakava, R. (2005). "Genes encoding enzymes of the lignin biosynthesis pathway in Eucalyptus". Genet. Mol. Biol. 28: 601–607.
- ↑ Thompson, D. C.; Barhoumi, R.; Burghardt, R. C. (1998). "Comparative toxicity of eugenol and its quinone methide metabolite in cultured liver cells using kinetic fluorescence bioassays". Toxicology and Applied Pharmacology. 149 (1): 55–63. doi:10.1006/taap.1997.8348. PMID 9512727.
- ↑ Fujisawa, S.; Atsumi, T.; Kadoma, Y.; Sakagami, H. (2002). "Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity". Toxicology. 177 (1): 39–54. doi:10.1016/S0300-483X(02)00194-4. PMID 12126794.
- ↑ "Eugenol Oil Overdose". New York Times Health Guide.
- ↑ Hartnoll, G.; Moore, D.; Douek, D. (1993). "Near fatal ingestion of oil of cloves". Archives of Disease in Childhood. 69 (3): 392–393. doi:10.1136/adc.69.3.392. PMC 1029532. PMID 8215554.
- ↑ "IFRA". www.ifraorg.org. Archived from the original on 2011-12-30.
- ↑ "Cropwatch Claims Victory Regarding "26 Allergens" Legislation" (PDF). www.leffingwell.com. Retrieved 2014-04-27.
- 1 2 3 Schmalz, Gottfried; Bindslev, Dorthe Arenholt (2008-10-10). Biocompatibility of Dental Materials. ISBN 9783540777823. Retrieved 2014-04-27.
- ↑ Bruynzeel, Derk P. (2014). "Balsam of Peru (Myroxylon pereirae)". Management of Positive Patch Test Reactions. Springer. pp. 53–55. doi:10.1007/978-3-642-55706-4_11. ISBN 978-3-540-44347-6. Retrieved 2014-04-27.
- ↑ Pathak, S. B.; Niranjan, K.; Padh, H.; Rajani, M. (2004). "TLC Densitometric Method for the Quantification of Eugenol and Gallic Acid in Clove". Chromatographia. 60 (3–4): 241–244. doi:10.1365/s10337-004-0373-y.
- 1 2 Bullerman, L. B.; Lieu, F. Y.; Seier, S. A. (July 1977). "Inhibition of growth and aflatoxin production by cinnamon and clove oils. Cinnamic aldehyde and eugenol". Journal of Food Science. 42 (4): 1107–1109. doi:10.1111/j.1365-2621.1977.tb12677.x.
- ↑ Lee, Kwang-Geun; Shibamoto, Takayuki (2001). "Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]". Food Chemistry. 74 (4): 443–448. doi:10.1016/S0308-8146(01)00161-3.
- ↑ Kreydiyyeh, S. I.; Usta, J.; Copti, R. (2000). "Effect of cinnamon, clove and some of their constituents on the Na+-K+-ATPase activity and alanine absorption in the rat jejunum". Food and Chemical Toxicology. 38 (9): 755–762. doi:10.1016/S0278-6915(00)00073-9. PMID 10930696.
- ↑ Dighe, V. V.; Gursale, A. A.; Sane, R. T.; Menon, S.; Patel, P. H. (2005). "Quantitative Determination of Eugenol from Cinnamomum tamala Nees and Eberm. Leaf Powder and Polyherbal Formulation Using Reverse Phase Liquid Chromatography". Chromatographia. 61 (9–10): 443–446. doi:10.1365/s10337-005-0527-6.
- ↑ Bennett, A.; Stamford, I. F.; Tavares, I. A.; Jacobs, S.; Capasso, F.; Mascolo, N.; Autore, G.; Romano, V.; Di Carlo, G. (1988). "The biological activity of eugenol, a major constituent of nutmeg (..Myristica fragrans..): Studies on prostaglandins, the intestine and other tissues". Phytotherapy Research. 2 (3): 124–130. doi:10.1002/ptr.2650020305.
- ↑ Johnson, C. B.; Kirby, J.; Naxakis, G.; Pearson, S. (1999). "Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.)". Phytochemistry. 51 (4): 507–510. doi:10.1016/S0031-9422(98)00767-5.
- ↑ Nakamura, C. V.; Ueda-Nakamura, T.; Bando, E.; Melo, A. F. N.; Cortez, D. A. G.; Dias Filho, B. P. (September 1999). "Antibacterial activity of Ocimum gratissimum L. essential oil" (PDF). Memórias do Instituto Oswaldo Cruz. 94 (5): 675–678. doi:10.1590/S0074-02761999000500022. PMID 10464416.
- ↑ Ize-Ludlow, D.; Ragone, S.; Bruck, I. S.; Bernstein, J. N.; Duchowny, M.; Peña, B. M. (2004). "Neurotoxicities in Infants Seen With the Consumption of Star Anise Tea". Pediatrics. 114 (5): e653–e656. doi:10.1542/peds.2004-0058. PMID 15492355.
- ↑ "Lemon balm". University of Maryland Medical Center. Retrieved 2010-12-07.
External links
- Kegley, S. E.; Hill, B. R.; Orme, S.; Choi, A. H. (2010). "Toxicity Information for Eugenol". PAN Pesticide Database,. San Francisco, CA: Pesticide Action Network, North America.
- "Eugenol (Mmm, dentist)". Molecule of the Day. ScienceBlogs. 4 October 2006. Archived from the original on 30 December 2009. Retrieved 7 December 2010.
